��Ŀ����
����Ŀ��(1)��������������Ʒ��
A. ʳ�� B. С�մ� C. ���� D. ʳ��(��Ҫ�ɷ�ΪCH3COOH)��
�ٴ���Ļ�ѧʽ��___________��
����Ҫ�ɷ�������ʽ�ε���___________(�����)��
��ˮ��Һ�ʼ��Ե���___________(�����)��
�ܴ�����ˮ�еĵ��뷽��ʽ��______________________��
(2)ѡ������ʵ�鷽��������������ʣ��뽫ʵ�鷽����������ں����� (�����)��
A. ��ȡ B. ���� C. ���� D. ����
�ٳ�ȥ������Һ�е���ɳ___________��
����������ˮ��ȡ����ˮ___________��
�۴ӵ�ˮ����ȡ��___________��
���𰸡�Na2CO3 B BC CH3COOH![]() CH3COO-+H+ B C A
CH3COO-+H+ B C A
��������
(1) �ٴ�����Ҫ�ɷ�Ϊ̼���ƣ���ѧʽ��Na2CO3��
��С�մ���Ҫ�ɷ�Ϊ̼�����ƣ�������ʽ�Σ��������B��
��A.NaCl��ǿ��ǿ���Σ����ƻ�ˮ�ĵ���ƽ�⣬ˮ��Һ������,A����
B.С�մ���NaHCO3������ǿ�������Σ�����Һ��HCO3-����ˮ�����ã�����ˮ���������H+���ƻ���ˮ�ĵ���ƽ�⣬����ʹ��Һ��c(OH-)>c(H+)��ˮ��Һ�ʼ��ԣ� B��ȷ��
C.������Na2CO3������ǿ�������Σ�����Һ��CO32-����ˮ�����ã�����ˮ���������H+���ƻ���ˮ�ĵ���ƽ�⣬����ʹ��Һ��c(OH-)>c(H+)��ˮ��Һ�ʼ��ԣ�C��ȷ��
D.ʳ����Ҫ�ɷ�ΪCH3COOH��������������ӣ�ʹ��Һ�����ԣ�D����
�ʺ���ѡ����BC��
��ʳ����Ҫ�ɷ�Ϊ���ᣬ������һԪ���ᣬ����Һ�д��ڵ���ƽ�⣬���뷽��ʽ�ǣ�CH3COOH![]() CH3COO-+H+��
CH3COO-+H+��
(2) ����ɳ������ˮ��ʳ��������ˮ��ʳ�ο�ѡ���˷����룬��ΪB��
������ˮ��ˮ�ķе�ͣ��������εķе�ߣ���ѡ���������ˮ����C��
�۵ⲻ������ˮ���������л��ܼ���ˮ�����ܼ��������ݣ��ʿ�ѡ��ȡ��������⣬��ΪA��

����Ŀ��������̼��ʯ�Ϳ��ɡ��˹����ꡢ�������л��ϳɵ��������Ź㷺��Ӧ�á�
(1)�״�����Ҫ�Ļ���ԭ�ϡ����úϳ���(CO��CO2��H2)�ڴ��������ºϳɼ״�����֪��Ӧ��صĻ�ѧ��������������:
��ѧ�� | H-H | C-O | C=O | H-O | C-H |
E/(KJ/mol) | 436 | 343 | 803 | 465 | 413 |
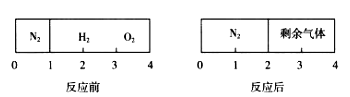
д��CO2��H2�ڴ�������������CH3OH(g)��H2O(g)���Ȼ�ѧ����ʽ________
(2)CH4��������CO2�Ĵ�ת��ԭ��ʾ��ͼ��ͼ��ʾ:

�ٹ��̢�,����1molH2ʱ����123.5kJ���������Ȼ�ѧ����ʽ��_______
�ڹ��̢�,ʵ���˺�Ȼ�����뺬̼���ֵķ��룬����H2O(g)�Ļ�ѧ����ʽ��________
����Ŀ��ij��ѧ��ȤС����ȡ����غ���ˮ�������й�̽��ʵ�顣
ʵ��һ����ȡ����غ���ˮ
������ͼ��ʾ��ʵ��װ�ý���ʵ�顣
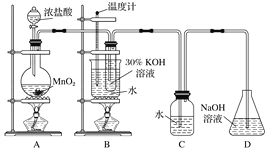
��1����ȡʵ�������ȡ��B���Թ���ȴ�ᾧ�����ˡ�ϴ�ӡ���ʵ�����������Ҫ����Ҫ�����������ձ�����������________________________��
��2�����Ե�B��Cװ�õ�λ�ã�________(���������� ��������)���B������صIJ��ʡ�
ʵ������������⻯�ط�Ӧ��̽��
��3���ڲ�ͬ������KClO3�ɽ�KI����ΪI2��KIO3����С�������ϵ��ʵ���о���Ӧ�����Է�Ӧ�����Ӱ�죬����ϵ��aʵ��ļ�¼������(ʵ���������½���)��
�Թܱ�� | 1 | 2 | 3 | 4 |
0.20 mol��L��1 KI/mL | 1.0 | 1.0 | 1.0 | 1.0 |
KClO3(s)/g | 0.10 | 0.10 | 0.10 | 0.10 |
6.0 mol��L��1 H2SO4/mL | 0 | 3.0 | 6.0 | 9.0 |
����ˮ/mL | 9.0 | 6.0 | 3.0 | 0 |
ʵ������ |
��ϵ��aʵ���ʵ��Ŀ����__________________________________________��
����2���Թ�ʵ������Ϊ����ɫ��Һ����ȡ��������Һ���������Һ����ɫ��������������Ψһ����ԭ����ΪKCl
ʵ�������ⶨ������ˮ����Ԫ�ص�����
��4����С����Ƶ�ʵ�鷽����ʹ����ͼװ�ã�����15.0 mL ������ˮ�������ⶨ���������������˷��������е���Ҫԭ����______________________________________��
(������ʵ��װ�ü�����ʧ���²����е�ԭ��)
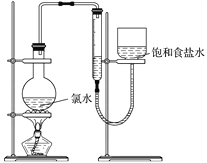
(5)�����������ϣ�Ϊ��С�����һ�����е�ʵ�鷽��(���������������̵�ϸ��)�� ______________��
���ϣ�i.��������ƻ����ָʾ����
ii.������������ɱ�SO2��H2O2��FeCl2�����ʻ�ԭ��Cl����