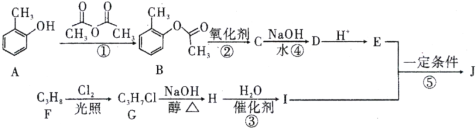
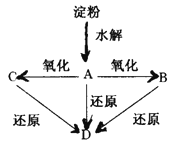
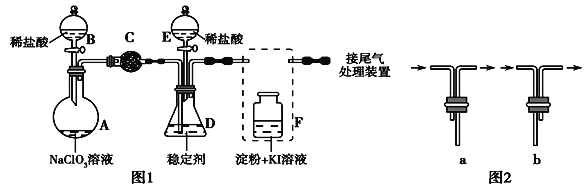
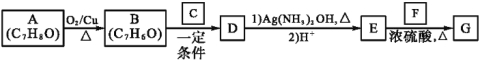
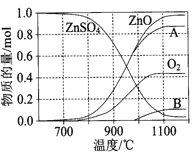
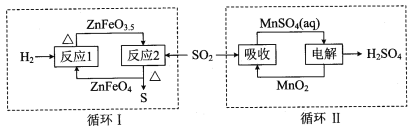
��Ŀ����
����Ŀ����֪X��Y��Z��W����Ԫ�طֱ���Ԫ�����ڱ����������������ڵ�Ԫ�أ���ԭ��������������X��Wͬ���壬Y��ZΪͬ���ڵ�����Ԫ�ء�Wԭ�ӵ�����������Y��Z ԭ�ӵ�����������֮�͡�Zԭ�������������Ǵ�����������3�������ƶϣ�
��1������Ԫ�������γɵĻ������У�����ˮ�Լ��Ե���̬�⻯��ĵ���ʽΪ ���������Ӽ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ �����м��Թ��ۼ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ ��
��2����X��Y��Z���γɵij������ӻ������� ��д��ѧʽ�����û�������[W������������Ӧˮ�����Ũ��Һ����ʱ��Ӧ�����ӷ���ʽΪ ��
���𰸡���1��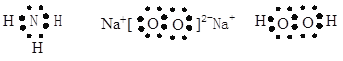
��2��NH4NO3NH![]() ��OH��
��OH�� ![]() NH3����H2O
NH3����H2O
���������������������Ԫ�������������Ķ����ڣ���X�ڵ�һ���ڣ�Ϊ��Ԫ�أ�W����ͬ���壬�ڵ�����ǰ��Ϊ��Ԫ�أ�Zԭ�������������Ǵ�����������3����Ϊ��Ԫ�أ�Wԭ�ӵ�����������Y��Z ԭ�ӵ�����������֮�ͣ���YΪ��Ԫ�ء���1�� ����Ԫ���������γɻ�������ֻ�а����Ǽ��Ե����壬�����ʽΪ��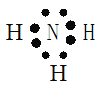 ���������Ӽ��ͷǼ��Թ��ۼ�������Ϊ�������ƣ������ʽΪ��
���������Ӽ��ͷǼ��Թ��ۼ�������Ϊ�������ƣ������ʽΪ�� �����м��Լ��ͷǼ��Լ�������Ϊ�������⣬�����ʽΪ��
�����м��Լ��ͷǼ��Լ�������Ϊ�������⣬�����ʽΪ�� �� ��2�������⡢���γɵ����ӻ�����Ϊ����泥������������Ƶ�Ũ��Һ����ʱ���ɰ��������ӷ���ʽΪ�� NH
�� ��2�������⡢���γɵ����ӻ�����Ϊ����泥������������Ƶ�Ũ��Һ����ʱ���ɰ��������ӷ���ʽΪ�� NH![]() ��OH��
��OH�� ![]() NH3����H2O��
NH3����H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�