��Ŀ����
��ҵ�Ƶõĵ�����(AlN)��Ʒ�г���������Al4C3��Al2O3��C�����ʡ�ijͬѧ���������ʵ��ֱ�ⶨ������(AlN)��Ʒ��AlN��Al4C3����������(����NH3��ǿ������Һ�е��ܽ�)��
(1)ʵ��ԭ��
��Al4C3�����ᷴӦ������CH4;
��AlN����ǿ���������,��������������Һ���ɰ���,��д��AlN��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ����������������������������������
(2)ʵ��װ��(��ͼ��ʾ)
(3)ʵ�����
������ʵ��װ��,����װ�õ������ԡ��Ƶ�Dװ�õ�����Ϊn g,�ζ��ܵĶ���Ϊa mL��
�ڳ�ȡm g AlN��Ʒ������ƿ��;���ý���,�رջ���������,����������,ͨ����Һ©���������������(�ѧʽ),����ƿ�����ʳ�ַ�Ӧ��
�۴���Ӧ������ȫ��,�رջ���������,����������,ͨ����Һ©���������������(�ѧʽ),����ƿ�����ʳ�ַ�Ӧ��
��������������������������(����ò�Ӧ���еIJ���)��
�ݼ�¼�ζ��ܵĶ���Ϊb mL,�Ƶ�Dװ�õ�����Ϊp g��
(4)���ݷ���
��AlN����������������������
������ȡ�ζ�������������ʱ,Һ������ҵ�,��������������������(�ƫ��ƫС������Ӱ�족)��
��Al4C3����������Ϊ��������������(��ʵ�������µ�����Ħ�����ΪVm)
(1)��AlN+NaOH+H2O NaAlO2+NH3��
NaAlO2+NH3��
(3)��K2��K3��K1��ϡ���ᡡ��K1��K3��NaOH���ܴ�K2,ͨ�����һ��ʱ��
(4)��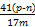 ��100%����ƫС����
��100%����ƫС����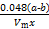 ��100%
��100%
����

ʵ������Ҫ������NaCl��Һ����ʵ���ҵ�NaCl�����������Na2SO4��NH4HCO3��ijͬѧ����������ͼ���ʵ���ȥ���ʣ��ش��������⣺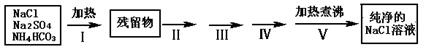
��1������I��ȥ�������ǣ��ѧʽ��_______________��ֱ�Ӽ���Ҫ���ڼ�ǿ����ٽ��м��ȣ������� ��
��2��������ͼ���ʵ����ƣ�����ص�ʵ�������ʵ�������ʵ��Ŀ����д���±��У�
| �������� | ʵ������ | ʵ��Ŀ�� |
| ����II�����������ܽ�õ���Һ�� | | |
| ����III�� |  | |
| ����IV�����ˣ�����Һ�� | | |
| ����V������Һ������� | 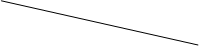 | |
��3�������õ�20���NaCl������Һ����֪20��ʱNaCl���ܽ��Ϊ36.0g��NaCl������Һ���ܶ�Ϊ1.12g/cm3 ����20���NaCl������Һ�����ʵ���Ũ��Ϊ mol/L��������������λ��Ч���֣���
���й�����Ӧ��Ʒ�к��е������������
| A������÷ζ�������ȣ��ʣ�һ�� ������������ |
| B������ˮ�ϰɣ��ȳ�����ζ�������Ҵ� |
| C�������˸��иƣ��������ˣ��Ȳ�ʹ�ˣ�����Ҳֱ�ˡ�����̼��� |
| D����Ҫ��Ƥ���ã������ô����������� |

 ��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��
��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg�� 2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��
2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��


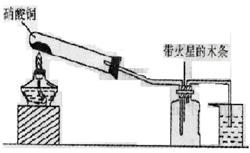


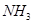 ��д����Ӧ�����ӷ���ʽ____________________________��
��д����Ӧ�����ӷ���ʽ____________________________��