جâؤ؟ؤعبف
،¾جâؤ؟،؟سذ¹ط´ك»¯¼ءµؤ´ك»¯»ْہيµبختجâ؟ة´س،°زز´¼´ك»¯رُ»¯تµرé،±µأµ½ز»ذ©بدت¶£®دآح¼تاؤ³»¯ر§ذثب¤ذ،×éةè¼ئµؤزز´¼´ك»¯رُ»¯µؤتµرé×°ضأ،£(جلت¾£؛ح¨³£سأذآضئµؤCu(OH)2ذü×از؛²ْةْש؛ىة«³ءµيہ´¼ىرéخïضتضذ؛¬سذب©»ù)،£
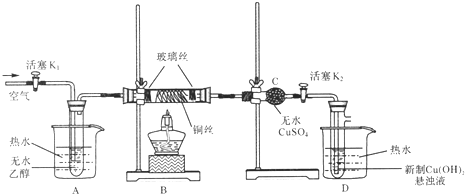
اë¾فح¼»ط´ًزشدآختجâ£؛
£¨1£©زائ÷ء¬½س°²×°حê±د£¬½ّذذتµرéا°بç؛خ¼ىرé×°ضأµؤئّأـذش£؟´ً£؛_____________£»
£¨2£©¶شAضذµؤزز´¼²ةسأث®ش،¼سببµؤؤ؟µؤتا____________£»
£¨3£©تµرéت±£¬µمب¼B´¦µؤ¾ئ¾«µئ؛َ£¬دب____________£¬شظ¼¯ضذ»ًرو¼سببحث؟£¬ثو؛َدٍ×°ضأضذ²»¶دµط»؛»؛¹ؤبë؟صئّ£¬´ثت±Bضذ¹غ²ىµ½µؤدضدَتا____________£¬·¢ةْµؤض÷زھ·´س¦µؤ»¯ر§·½³جت½خھ____________£¬µ±·´س¦½ّذذز»¶خت±¼ن؛َ£¬زئب¥¾ئ¾«µئ£¬¼جذّ²»¶د»؛»؛µط¹ؤبë؟صئّ£¬Bضذبشضط¸´بçةددضدَ£¬ثµأ÷B´¦·¢ةْµؤ·´س¦تاز»¸ِ____________·´س¦(جî،°خüبب،±»ٍ،°·إبب،±)£®
£¨4£©×°ضأCµؤ×÷سأتا____________£¬ؤـشع´ث´¦¹غ²ىµ½µؤدضدَتا____________£»
£¨5£©×°ضأDضذµؤدضدَتا____________،£
،¾´ً°¸،؟
£¨1£©¹ط±ص»îبûK2£¬´ٍ؟ھK1£¬µمب¼B´¦¾ئ¾«µئ£¬Aضذز؛جهر¹ب볤¹ـضذ£»ح£ض¹¼سببہنب´ضءتزخآ£¬Aضذ»ض¸´ش×´(»ٍ¹ط±صK1£¬´ٍ؟ھK2£¬µمب¼B´¦¾ئ¾«µئ£¬Dضذ³¤¹ـ؟عأ°ئّإف£»ح£ض¹¼سببہنب´ضءتزخآ£¬Dضذز؛جهµ¹خüب볤¹ـذخ³ةز»¶خث®ضù)£¬ثµأ÷ئّأـذشء¼؛أ£»
£¨2£©خھت¹Aضذزز´¼½د³¤ت±¼نµطئ½خبµطئّ»¯³ةزز´¼صôئّ£»
£¨3£©ش¤ببض±²£ء§¹ـ حث؟سة؛ىة«±نخھ؛عة«£¬؛ـ؟ىسض±نخھ؛ىة«£¬2CH3CH2OH+O2![]() 2CH3CHO+2H2O ·إبب£»
2CH3CHO+2H2O ·إبب£»
£¨4£©¼ىرéزز´¼رُ»¯²ْةْµؤH2O °×ة«·غؤ©±نخھہ¶ة«¾§جه£»
£¨5£©²ْةْש؛ىة«³ءµي
،¾½âخِ،؟
تشجâ·ضخِ£؛£¨1£©×°ضأئّأـذش¼ىرéµؤشہيتا£؛ح¨¹ئّجه·¢ةْئ÷سëز؛جه¹¹³ة·â±صجهدµ£¬زہ¾ف¸ؤ±نجهدµؤعر¹ا؟ت±²ْةْµؤدضدَ(بçئّإفµؤةْ³ة،¢ث®ضùµؤذخ³ة،¢ز؛أوµؤة½µµب)ہ´إذ¶د×°ضأئّأـذشµؤ؛أ»µ£¬·½·¨خھ£؛¹ط±ص»îبûK2£¬´ٍ؟ھK1£¬µمب¼B´¦¾ئ¾«µئ£¬Aضذز؛جهر¹ب볤¹ـضذ£»ح£ض¹¼سببہنب´ضءتزخآ£¬Aضذ»ض¸´ش×´(»ٍ¹ط±صK1£¬´ٍ؟ھK2£¬µمب¼B´¦¾ئ¾«µئ£¬Dضذ³¤¹ـ؟عأ°ئّإف£»ح£ض¹¼سببہنب´ضءتزخآ£¬Dضذز؛جهµ¹خüب볤¹ـذخ³ةز»¶خث®ضù)£¬ثµأ÷ئّأـذشء¼؛أ£»¹ت´ً°¸خھ£؛¹ط±ص»îبûK2£¬´ٍ؟ھK1£¬µمب¼B´¦¾ئ¾«µئ£¬Aضذز؛جهر¹ب볤¹ـضذ£»ح£ض¹¼سببہنب´ضءتزخآ£¬Aضذ»ض¸´ش×´(»ٍ¹ط±صK1£¬´ٍ؟ھK2£¬µمب¼B´¦¾ئ¾«µئ£¬Dضذ³¤¹ـ؟عأ°ئّإف£»ح£ض¹¼سببہنب´ضءتزخآ£¬Dضذز؛جهµ¹خüب볤¹ـذخ³ةز»¶خث®ضù)£¬ثµأ÷ئّأـذشء¼؛أ£»
£¨2£©¶شAضذµؤزز´¼²ةسأث®ش،¼سببµؤؤ؟µؤتاخھت¹Aضذزز´¼½د³¤ت±¼نµطئ½خبµطئّ»¯³ةزز´¼صôئّ£»¹ت´ً°¸خھ£؛خھت¹Aضذزز´¼½د³¤ت±¼نµطئ½خبµطئّ»¯³ةزز´¼صôئّ£»
£¨3£©زز´¼´ك»¯رُ»¯¹³جضذ£¬حدبسëرُئّ¼سبب·´س¦ةْ³ةرُ»¯ح£¬رُ»¯حشظسëزز´¼·´س¦ةْ³ةززب©؛حح،¢ث®£¬ثùزشتµرéت±£¬µمب¼B´¦µؤ¾ئ¾«µئ؛َ£¬دبش¤ببض±²£ء§¹ـ£¬شظ¼¯ضذ»ًرو¼سببحث؟£¬؟´µ½µؤدضدَخھ£؛ث؟سة؛ىة«±نخھ؛عة«£¬؛ـ؟ىسض±نخھ؛ىة«£¬·´س¦·½³جت½£؛2CH3CH2OH+O2![]() 2CH3CHO+2H2O£¬زئب¥¾ئ¾«µئ£¬¼جذّ²»¶د»؛»؛µط¹ؤبë؟صئّ£¬Bضذبشضط¸´بçةددضدَ£¬ثµأ÷¸أ·´س¦خھ·إبب·´س¦£¬·إ³ِµؤببء؟ؤـ¹»خ¬³ض·´س¦µؤ½ّذذ£»¹ت´ً°¸خھ£؛ش¤ببض±²£ء§¹ـ حث؟سة؛ىة«±نخھ؛عة«£¬؛ـ؟ىسض±نخھ؛ىة«£¬2CH3CH2OH+O2
2CH3CHO+2H2O£¬زئب¥¾ئ¾«µئ£¬¼جذّ²»¶د»؛»؛µط¹ؤبë؟صئّ£¬Bضذبشضط¸´بçةددضدَ£¬ثµأ÷¸أ·´س¦خھ·إبب·´س¦£¬·إ³ِµؤببء؟ؤـ¹»خ¬³ض·´س¦µؤ½ّذذ£»¹ت´ً°¸خھ£؛ش¤ببض±²£ء§¹ـ حث؟سة؛ىة«±نخھ؛عة«£¬؛ـ؟ىسض±نخھ؛ىة«£¬2CH3CH2OH+O2![]() 2CH3CHO+2H2O ·إبب£»
2CH3CHO+2H2O ·إبب£»
£¨4£©ءٍثلحخھ°×ة«¹ججه£¬¼«ز×½ل؛دث®ةْ³ةہ¶ة«µؤخهث®ءٍثلح£¬ح¨³£سأ´ث¼ىرéث®µؤ´وشع£»¹ت´ً°¸خھ£؛¼ىرéزز´¼رُ»¯²ْةْµؤH2O °×ة«·غؤ©±نخھہ¶ة«¾§جه£»
£¨5£©ززب©؛¬سذب©»ùؤـ¹»سëذآضئµؤاâرُ»¯ح·´س¦ةْ³ةש؛ىة«µؤرُ»¯راح،¢ززثل؛حث®£¬ثùزش؟´µ½µؤدضدَخھ£؛²ْةْש؛ىة«³ءµي£»¹ت´ً°¸خھ£؛²ْةْש؛ىة«³ءµي

 ز»±¾؛أجâ؟عثمجâ؟¨دµءذ´ً°¸
ز»±¾؛أجâ؟عثمجâ؟¨دµءذ´ً°¸،¾جâؤ؟،؟![]() ت±£¬2Lأـ±صبفئ÷ضذ³نبë
ت±£¬2Lأـ±صبفئ÷ضذ³نبë![]()
![]() £¬·¢ةْ·´س¦£؛
£¬·¢ةْ·´س¦£؛![]() £¬»ٌµأبçدآت¾ف£؛دآءذإذ¶دصب·µؤتا
£¬»ٌµأبçدآت¾ف£؛دآءذإذ¶دصب·µؤتا![]()
ت±¼ن | 0 | 20 | 40 | 60 | 80 | 100 |
|
|
|
|
|
|
|
A.![]() ؤع£¬
ؤع£¬![]()
B.شعدàح¬جُ¼دآ£¬؟ھت¼ت±بôدٍبفئ÷ضذ³نبëµؤتا![]()
![]() £¬´ïµ½ئ½؛â؛َ
£¬´ïµ½ئ½؛â؛َ![]() µؤ×ھ»¯آتخھ
µؤ×ھ»¯آتخھ![]()
C.·´س¦´ïئ½؛âت±£¬خüتصµؤببء؟خھ![]() kJ
kJ
D.100sت±شظح¨بë![]() mol
mol![]() £¬´ïذآئ½؛âت±
£¬´ïذآئ½؛âت±![]() µؤ×ھ»¯آتشِ´َ
µؤ×ھ»¯آتشِ´َ
،¾جâؤ؟،؟£¨¢ٌ£©ؤ³»¯ر§·´س¦A![]() B+Cشعبضض²»ح¬جُ¼دآ½ّذذ£¬B،¢Cµؤئًت¼إ¨¶بخھ0£¬·´س¦خïAµؤإ¨¶ب£¨mol/L£©ثو·´س¦ت±¼ن£¨min£©µؤ±ن»¯اé؟ِبçدآ±يثùت¾£¬±يضذخآ¶بخھةمتد¶ب£¨،و£©،£
B+Cشعبضض²»ح¬جُ¼دآ½ّذذ£¬B،¢Cµؤئًت¼إ¨¶بخھ0£¬·´س¦خïAµؤإ¨¶ب£¨mol/L£©ثو·´س¦ت±¼ن£¨min£©µؤ±ن»¯اé؟ِبçدآ±يثùت¾£¬±يضذخآ¶بخھةمتد¶ب£¨،و£©،£
تµرé ذٍ؛إ | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
1 | 800 | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
2 | 800 | 1.0 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
3 | 820 | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 | 0.20 |
£¨1£©¸أ·´س¦تôسع__________·´س¦£¨جî،°؟ةؤو،±»ٍ،°²»؟ةؤو،±£©،£
£¨2£©شعتµرé1،¢2ضذ£¬سذز»¸ِتµرéت¹سأءث´ك»¯¼ء،£اëؤمہûسأ±يضذت¾فإذ¶دتµرé___£¨جî1»ٍ2£©ت¹سأءث´ك»¯¼ء£¬ہيسةتا_______________،£
£¨3£©¸أتµرé±يأ÷£¬س°دى»¯ر§·´س¦ثظآتµؤزٍثط»¹سذ__________،£
£¨II£©دآح¼تا1molNO2؛ح1molCO·´س¦ةْ³ة1molCO2؛ح1molNO¹³جضذؤـء؟±ن»¯ت¾زâح¼£¬اëذ´³ِNO2؛حCO·´س¦µؤبب»¯ر§·½³جت½£؛__________________،£

¸أ·´س¦µؤ»î»¯ؤـµبسع____________________

