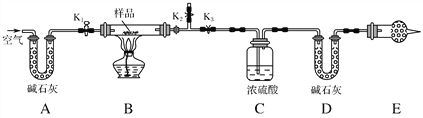
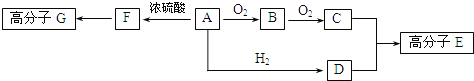
��Ŀ����
����Ŀ���״�����Ϊ2l���͵�����ȼ�ϣ���ҵ��ͨ�����з�Ӧ�ٺ͢ڣ���CH4��H2OΪԭ�����Ʊ��״���
�� CH4(g)+H2O(g) ![]() CO(g)+3H2(g) ��H1
CO(g)+3H2(g) ��H1
�� CO(g)+2H2(g) ![]() CH3OH(g) ��H2
CH3OH(g) ��H2
��0.20mol CH4��0.30 mol H2O(g)ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��

��1����֪��P1��100��ʱ�ﵽƽ�������ʱ��Ϊ5min������CH4��ʾ��ƽ����Ӧ����Ϊ______��
��2����Ӧ�ٵġ�H1____0��ͼ�е�P1______P2���<������=����>������
��3����ѹǿΪ0.1MPa�����£���һ����CO��H2�Ļ�������ڴ������������Է���Ӧ���ɼ״�����Ӧ�ڵġ�H2____0����S____0���<������=����>������
��4���������и��������ݣ����㷴Ӧ����100��ʱ��ƽ�ⳣ��ֵ____________��д��������̼������
���𰸡� 0.002mol/(L��min) > < < < 1.35��10-3(mol/L)2
����������1��100��ʱ�ﵽƽ��ʱ�����ת����Ϊ0.5�������μӷ�Ӧ�����ʵ���=0.2mol��0.5=0.1mol��v��CH4��=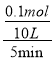 =0.002mol/(L��min)
=0.002mol/(L��min)
��2����ͼ��������¶�����ʱ�����ת��������ƽ�������ƶ�����Ӧ���ȣ���H��0����ͼ��֪�¶���ͬʱ������ƽ��ʱ��ѹǿΪP1��CH4ת���ʸߣ�ƽ��������Ӧ�����ƶ�����ӦΪ�����������ķ�Ӧ����Сѹǿƽ�����������ķ����ƶ�����P1��P2��
��3����ѹǿΪ0.1MPa�����£���һ����CO��H2�Ļ�������ڴ������������Է���Ӧ���ɼ״�����Ӧ������������٣�����S<0���ַ�Ӧ���Է����У���H<T��S<0��
��4����Ӧ����100��ʱ��c(CH4)=0.2mol��0.5/10L=0.01mol/L��c(H2O)=��0.3mol�D0.1mol��/10L=0.02mol/L��c(CO)=��C(CH4)=0.01mol/L��c()=3��C(CH4)=3��0.01mol/L=0.03mol/L��K=![]() =1.35��10-3(mol/L)2
=1.35��10-3(mol/L)2

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�