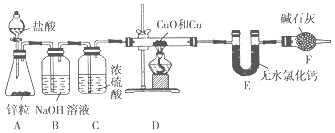
��Ŀ����
����Ŀ�������л���֮���ת����ϵ�Լ�ת��������Է��������仯��ϵ���£�
����ת����ϵ��RCH2OH ![]() RCHO
RCHO![]() RCOOH
RCOOH
��Է���������M M2M+14
��֪������A��ֻ����C.H��O����Ԫ�أ�һ���������ܷ���������Ӧ������C����Է�������Ϊ104.A����������֮���ת����ϵ��ͼ��ʾ��
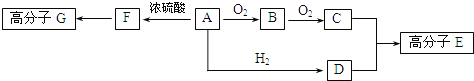
��ش��������⣺
(1)F�к��еĹ�������________��G�Ľṹ��ʽ___________��
(2)һ��������B����������Ӧ�Ļ�ѧ����ʽ__________��
(3)��C.D��һ�������·�Ӧ�������ɻ�״������仯ѧ��Ӧ����ʽΪ_________��
(4)A��ͬ���칹��ܶ࣬д����A�����������Ų�ͬ��������NaOH��Һ��Ӧ��2��ͬ���칹��Ľṹ��ʽ��_______________________��
���𰸡� ̼̼˫����ȩ�� ![]() OHC-CH2-CHO+4Ag��NH3��2OH
OHC-CH2-CHO+4Ag��NH3��2OH![]() 4Ag+NH4OOC-CH2-COONH4+6NH3+2H2O HOCH2CH2CH2OH+HOOCCH2COOH
4Ag+NH4OOC-CH2-COONH4+6NH3+2H2O HOCH2CH2CH2OH+HOOCCH2COOH![]()
![]() +2H2O CH3CH2COOH��CH3COOCH3
+2H2O CH3CH2COOH��CH3COOCH3
������������A��ֻ����C��H��O����Ԫ�أ�һ���������ܷ���������Ӧ����A�к�-CHO��A�ɷ�������������Ӧ����A�л���-OH����CӦΪ��Ԫ�ᣬC����Է�������Ϊ104��104-(45��2)=14��������1��CH2������AΪHOCH2CH2CHO��BΪOHC-CH2-CHO��CΪHOOC-CH2-COOH��DΪHOCH2CH2CH2OH��C��D�������۷�Ӧ���ɸ߷��ӣ�FΪCH2=CHCHO��F�����Ӿ۷�Ӧ���ɸ߷��ӡ�
(1)FΪCH2=CHCHO��������Ϊ̼̼˫����ȩ����F�����Ӿ۷�Ӧ����GΪ![]() ���ʴ�Ϊ��̼̼˫����ȩ����
���ʴ�Ϊ��̼̼˫����ȩ����![]() ��
��
(2)B����������Ӧ�Ļ�ѧ����ʽΪOHC-CH2-CHO+4Ag(NH3)2OH![]() 4Ag+NH4OOC-CH2-COONH4+6NH3+2H2O���ʴ�Ϊ��OHC-CH2-CHO+4Ag(NH3)2OH
4Ag+NH4OOC-CH2-COONH4+6NH3+2H2O���ʴ�Ϊ��OHC-CH2-CHO+4Ag(NH3)2OH![]() 4Ag+NH4OOC-CH2-COONH4+6NH3+2H2O��
4Ag+NH4OOC-CH2-COONH4+6NH3+2H2O��
(3)C��D��һ�������·�Ӧ�������ɻ�״������Ļ�ѧ��Ӧ����ʽΪHOCH2CH2CH2OH+HOOCCH2COOH![]()
![]() +2H2O���ʴ�Ϊ��HOCH2CH2CH2OH+HOOCCH2COOH
+2H2O���ʴ�Ϊ��HOCH2CH2CH2OH+HOOCCH2COOH![]()
![]() +2H2O��
+2H2O��
(4)AΪHOCH2CH2CHO����A�����������Ų�ͬ��������NaOH��Һ��Ӧ��ͬ���칹��ΪCH3CH2COOH��CH3COOCH3��HCOOCH2CH3���ʴ�Ϊ��CH3CH2COOH��CH3COOCH3��