��Ŀ����
����Ŀ���Ĵ�ʢ���屶�ӡ����屶��Ϊԭ�Ͽ����Ƶû�����A��A�Ľṹ��ʽ����ͼ��ʾ��

��ش��������⣺
��1��A�ķ���ʽ��_________��
��2���л�������A������������¼��ȷ���ˮ�ⷴӦ�ɵõ�B����д��B�Ľṹ��ʽ_________��
��3���л�������C�Ǻϳ�����������ҩ���ԭ��֮һ��C���Կ�����B�����������ʵ���֮��1:2�����ӳɷ�Ӧ�õ��IJ��C�ṹ��Ϊ�ȶ�������ʹ��ˮ��ɫ����д��C����ˮ��Ӧ�Ļ�ѧ����ʽ_________��
���𰸡�C14H10O9 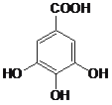
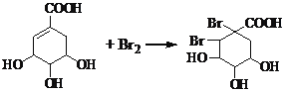
��������
�����л���A�Ľṹ��ʽ��Ϸ����к��еĹ����ŵ����ʷ������
��1�����ݽṹ��ʽ��֪A�ķ���ʽ��C14H10O9��
��2���л�������A����������������������¼��ȷ���ˮ�ⷴӦ�ɵõ�B��B�Ľṹ��ʽΪ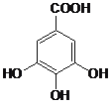 ��
��
��3���л�������C�Ǻϳ�����������ҩ���ԭ��֮һ��C���Կ�����B�����������ʵ���֮��1:2�����ӳɷ�Ӧ�õ��IJ��C�ṹ��Ϊ�ȶ�������ʹ��ˮ��ɫ��˵��C�����к���̼̼˫�������ǻ���������̼̼˫���ϣ����C����ˮ��Ӧ�Ļ�ѧ����ʽΪ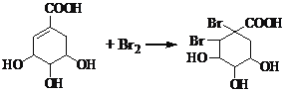 ��
��

����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������·�Ӧ��2N2O5(g) ![]() 4NO2(g)��O2(g)��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
4NO2(g)��O2(g)��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
ʱ��/s | 0 | 500 | 1000 | 1500 |
c(N2O5)mol/L | 5.00 | 3.53 | 2.50 | 2.50 |
����˵���в���ȷ���ǣ�
A. T1�¶��£�500sʱO2��Ũ��Ϊ0.74mol/L
B. ƽ������������䣬�����������ѹ����ԭ����1/2������ƽ��ʱc(N2O5)>5.00 mol/L
C. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1<K2
D. T1�¶��µ�ƽ�ⳣ��ΪK1=125��ƽ��ʱN2O5��ת����Ϊ0.5