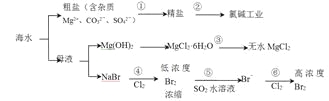
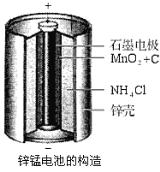
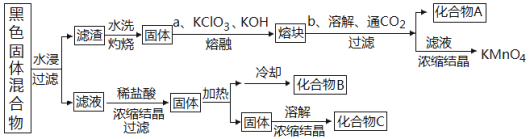
МвДҝДЪИЭ
ЎҫМвДҝЎҝ»ШҙрПВБРОКМвЈә
ЈЁ1Ј©Ҫ«өИОпЦКөДБҝөДAәНBЈ¬»мәПУЪ2 LөДГЬұХИЭЖчЦРЈ¬·ўЙъИзПВ·ҙУҰЈә3A(g) Ј« B(g) ![]() xC(g) Ј«2D(g),5minәуІвөГc(D)ЈҪ0.5molЎӨLЈӯ1Ј¬c(A)ЎГc(B)ЈҪ3ЎГ5Ј¬CөД·ҙУҰЛЩВККЗ0.1molЎӨLЈӯ1ЎӨminЈӯ1ЎЈ
xC(g) Ј«2D(g),5minәуІвөГc(D)ЈҪ0.5molЎӨLЈӯ1Ј¬c(A)ЎГc(B)ЈҪ3ЎГ5Ј¬CөД·ҙУҰЛЩВККЗ0.1molЎӨLЈӯ1ЎӨminЈӯ1ЎЈ
ўЩAФЪ5minД©өДЕЁ¶ИКЗ____________ЎЈ
ўЪv(B)ЈҪ____________ЎЈ
ўЫxЈҪ____________ЎЈ
ЈЁ2Ј©ФЪ25ЎжКұЈ¬Пт100mLә¬ВИ»ҜЗв14.6gөДСОЛбИЬТәЦРЈ¬·ЕИл5.6gҙҝМъ·ЫЈ¬·ҙУҰҪшРРөҪ2minД©КХјҜөҪЗвЖш1.12 L(ұкЧјЧҙҝц)Ј¬ФЪҙЛәуУЦҫӯ№э4minЈ¬Мъ·ЫНкИ«ИЬҪвЎЈИфІ»ҝјВЗИЬТәМе»эөДұд»ҜЈ¬ФтЈә
ўЩЗ°2minДЪУГFeCl2ұнКҫөДЖҪҫщ·ҙУҰЛЩВККЗ______________ЎЈ
ўЪәу4minДЪУГHClұнКҫөДЖҪҫщ·ҙУҰЛЩВККЗ________________ЎЈ
ўЫЗ°2minУләу4minПаұИЈ¬·ҙУҰЛЩВК__________ҪПҝмЈ¬ЖдФӯТтКЗ__________________ЎЈ
Ўҫҙр°ёЎҝЈЁ1Ј©ўЩ0.75molЎӨLЈӯ1Ј»ўЪ0.05molЎӨLЈӯ1ЎӨminЈӯ1Ј» ўЫ 2Ј»
ЈЁ2Ј©ўЩ0.25molЎӨ(LЎӨmin)Јӯ 1Ј» ўЪ0.25molЎӨ(LЎӨmin)ЈӯЈ»ўЫЗ°2minөДЖҪҫщ·ҙУҰЛЩВКҙуУЪәу4minөДЖҪҫщ·ҙУҰЛЩВКЈ»ФЪЖдЛыМхјюІ»ұдКұЈ¬Фцҙу·ҙУҰОпөДЕЁ¶ИЈ¬·ҙУҰЛЩВКФцҙуЈ¬ЛжЧЕ·ҙУҰҪшРРЈ¬·ҙУҰОпөДЕЁ¶ИЦрҪҘјхРЎЈ¬Тт¶шvЛжЦ®јхРЎЎЈ
ЎҫҪвОцЎҝ
КФМв·ЦОцЈәЈЁ1Ј©ўЩ 3A(g)Ј«B(g) ![]() xC(g)Ј«2D(g)
xC(g)Ј«2D(g)
ЖрКјЈЁЕЁ¶ИЈ©Јә a a 0 0
ПыәДЈЁЕЁ¶ИЈ©Јә 0.75 0.25 0.5
ЖҪәвЈЁЕЁ¶ИЈ©Јә(aЈӯ0.75) (aЈӯ0.25) 0.5
ёщҫЭc(A)Јәc(B)=3Јә5Ј¬ҪвөГa=1.5molЎӨLЈӯ1Ј¬ТтҙЛ·ҙУҰәуc(A)=(1.5Јӯ0.75)molЎӨLЈӯ1=0.75molЎӨLЈӯ1Ј»ўЪёщҫЭ·ҙУҰЛЩВКөД¶ЁТеЈ¬v(B)=0.25/5mol/(LЎӨmin)=0.05 mol/(LЎӨmin)Ј»ўЫёщҫЭ»ҜС§ЛЩВКЦ®ұИөИУЪ»ҜС§јЖБҝКэЦ®ұИЈ¬x=2Ј»ЈЁ2Ј©ўЩFeЈ«2HCl=FeCl2Ј«H2ЎьЈ¬n(FeCl2)=n(H2)=1.12/22.4mol=0.05molЈ¬v(FeCl2)=0.05/(2ЎБ100ЎБ10Јӯ3)mol/(LЎӨmin)=0.25 mol/(LЎӨmin)Ј»ўЪЗ°2minПыәДөДFeөДОпЦКөДБҝОӘ1.12/22.4mol=0.05molЈ¬әу4minПыәДn(HCl)=2ЎБЈЁ5.6/56Јӯ0.05Ј©mol=0.1molЈ¬v(HCl)=0.1/(4ЎБ100ЎБ10Јӯ3) mol/(LЎӨmin)=0.25 mol/(LЎӨmin)Ј»ўЫЖдЛыМхјюІ»ұдЈ¬ЛжЧЕ·ҙУҰөДҪшРРСОЛбөДЕЁ¶ИҪөөНЈ¬·ҙУҰЛЩВКјх»әЈ¬З°2minөДЖҪҫщ·ҙУҰЛЩВКҙуУЪәу4minөДЖҪҫщ·ҙУҰЛЩВКЎЈ

 П°Мвҫ«СЎПөБРҙр°ё
П°Мвҫ«СЎПөБРҙр°ёЎҫМвДҝЎҝA~HҫщОӘ¶МЦЬЖЪФӘЛШЈ¬A~FФЪФӘЛШЦЬЖЪұнЦРөДПа¶ФО»ЦГИзПВНјЛщКҫЈ¬GУлЖдЛьЖЯЦЦФӘЛШІ»ФЪН¬Т»ЦЬЖЪЈ¬HКЗ¶МЦЬЖЪЦРФӯЧУ°лҫ¶ЧоҙуөДЦчЧеФӘЛШЎЈУЙBЎўGЧйіЙөДЖшМ¬»ҜәПОпјЧЛ®ИЬТәіКјоРФЎЈ
A | B | C | |
D | E | F |
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©РҙіцјЧөДөзЧУКҪЈ¬КөСйКТЦЖИЎЖшМејЧөД»ҜС§·ҪіМКҪОӘЎЈ
ЈЁ2Ј©BЎўCЎўGёцКэұИОӘ1:1:5РОіЙөД»ҜәПОпөД»ҜС§јьАаРНОӘЎЈ
AЈ®АлЧУјь
BЈ®ј«РФјь
CЈ®·Зј«РФјь
ЈЁ3Ј©ЗлУГөзЧУКҪұнКҫAE2өДРОіЙ№эіМЎЈ
ЈЁ4Ј©УГАлЧУ·ыәЕұнКҫCЎўEЎўFЎўHЛДЦЦАлЧУөД°лҫ¶УЙҙуөҪРЎөДЛіРтЎЈ
ЈЁ5Ј©УГТ»ёцАлЧУ·ҪіМКҪҪвКНAұИD·ЗҪрКфРФЗҝөДФӯТтЎЈ