��Ŀ����
13��ŵ����������ҽѧ�������һ��ʧ����1948����������ʿ��ѧ�����գ��������շ����˾綾�л���ɱ���DDT����õ���ŵ������������������DDT��һ���ѽ���Ļ�������Բ���ʱ�䳤��������������������ֹ������ʹ�ã�DDT�Ľṹ��ʽΪ �����й���DDT��˵������ȷ���ǣ���������
�����й���DDT��˵������ȷ���ǣ���������| A�� | DDT�����������������ܹ�ƽ�� | |
| B�� | DDT�������ڷ����� | |
| C�� | ��ʹԭ�����ϵ�������ԭ��������ͬһ�������ϣ�����6��ͬ���칹�� | |
| D�� | DDT���Խ����ԭ����DDT���Է���ˮ�ⷴӦ |
���� A�����ݱ���������ԭ�ӹ�ƽ����з���DDT��������������ƽ�������
B������DDT�����д�����ԭ�ӣ����������ࣻ
C����ʹԭ�����ϵ�������ԭ��������ͬһ�������ϣ���Ӧ��ͬ���칹��Ϊλ���칹��
D��DDT�����к�����ԭ�ӣ��ܹ�����ˮ�ⷴӦ��
��� �⣺A�����ڱ���������ԭ�ӹ�ƽ�棬����DDT�������������Թ�ƽ�棬��A����
B��DDT�����к��е�ԭ�ӳ���C��H���⣬��������ԭ�ӣ����Բ����ڷ���������B����
C����ʹԭ�����ϵ�������ԭ��������ͬһ�������ϣ�����Clԭ�ӿ��ܵ�λ����ͼ ��Ӧ�IJ�ͬλ����1��2��1��3��1��4��1��5��2��3��2��4����6��ͬ���칹�壬��C��ȷ��
��Ӧ�IJ�ͬλ����1��2��1��3��1��4��1��5��2��3��2��4����6��ͬ���칹�壬��C��ȷ��
D��DDT�����к�����ԭ�ӣ�һ���������ܹ�����ˮ�ⷴӦ����D����
��ѡC��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ�߿��������ͣ���Ŀ�Ѷ��еȣ�������Ϣ�����ʵĽṹ������±����������Ϊ���Ĺؼ�����ȷ��ѧ�뻷��������ũҩʹ�õĹ�ϵ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
1��NH3�ʹ�����O2��һ�������·�����Ӧ��4NH3��g��+3O2��g��?2N2��g��+6H2O��g������һ�ݻ������2L�ܱ������г���4mol NH3��3mol O2��4min������ɵ�H2Oռ������������40%�������б�ʾ�˶�ʱ���ڸ÷�Ӧ��ƽ�����ʵ�ʽ�ӣ���ȷ���ǣ�������
| A�� | v��N2���T0.225 mol/��L•min�� | B�� | v��H2O���T0.375 mol/��L•min�� | ||
| C�� | v��O2���T0.225 mol/��L•min�� | D�� | v��NH3���T0.450 mol/��L•min�� |
8�����и��黯�����У���ѧ�������й�������ȷ���ǣ�������
| A�� | CaCl2��Na2O2�ж�ֻ�������Ӽ� | |
| B�� | NaOH��NaHS���Ⱥ������Ӽ����ֺ��м��Լ� | |
| C�� | CO2��H2S�ж�ֻ���зǼ��Լ� | |
| D�� | H2O2��CS2���Ⱥ��м��Լ����ֺ��зǼ��Լ� |
5������װ������ԭ��ص��ǣ�������
| A�� | 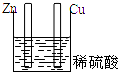 | B�� | 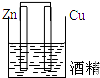 | C�� | 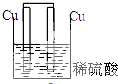 | D�� | 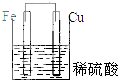 |
2��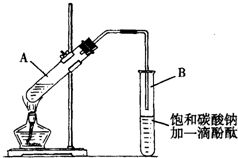 ��֪�������ݣ�
��֪�������ݣ�
ѧ����ʵ������ȡ������������Ҫ�������£�
����30mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㣮
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1��д����ȡ���������Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����BC
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ����������Ҵ��Ļӷ������ٸ���Ӧ�ķ�����
��4��ָ����������۲쵽�������Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��
 ��֪�������ݣ�
��֪�������ݣ�| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g•cm-3�� |
| �ҡ��� | -117.0 | 78.0 | 0.79 |
| �ҡ��� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | -- | 338.0 | 1.84 |
����30mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㣮
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1��д����ȡ���������Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ����BC
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ����������Ҵ��Ļӷ������ٸ���Ӧ�ķ�����
��4��ָ����������۲쵽�������Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��
3����ˮϡ��0.1mol•L-1��ˮʱ����Һ����ˮ�������Ӷ���С���ǣ�������
| A�� | $\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$ | B�� | $\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$ | C�� | n��OH-����c��H+�� | D�� | c��H+����c��OH-���ij˻� |

 ���������۷�Ӧ����Ľṹ��ʽΪ
���������۷�Ӧ����Ľṹ��ʽΪ ��P���ʵ��������ʵ�����������Ϊ����ȩQ����Q��������Һ������Ӧ�Ļ�ѧ����ʽΪ
��P���ʵ��������ʵ�����������Ϊ����ȩQ����Q��������Һ������Ӧ�Ļ�ѧ����ʽΪ ��
��
 ��A��B���Է������Ƣٵķ�Ӧ�����л������÷�Ӧ�Ļ�ѧ����ʽΪ
��A��B���Է������Ƣٵķ�Ӧ�����л������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��