��Ŀ����
��������泥�NH2COONH4����һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȡ�ij��ѧ��ȤС��ģ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�
2NH3(g)+CO2(g) NH2COONH4(s) + Q (Q > 0 )
NH2COONH4(s) + Q (Q > 0 )
��1��������ͼװ����ȡ����������ѡ����Լ��� ��
�Ʊ���������淋�װ������ͼ��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�С� ��������϶�ʱ��ֹͣ�Ʊ���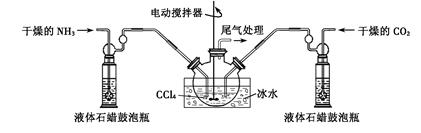
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʡ�
��2���������ñ�ˮ��ȴ��ԭ����___________ __ _��
��3��Һ��ʯ������ƿ��������_______��
��4���ӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽����_______����д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����________����дѡ����ţ���
a. ��ѹ���Ⱥ�� b. ��ѹ���Ⱥ�� c. ���40 �����º��
��5��β������װ����ͼ��ʾ��˫ͨ�����ܵ����ã�________ ��Ũ��������ã� ��______________ _��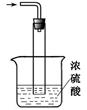
��6��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7820 g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000 g������Ʒ�а�������淋����ʵ�������Ϊ
___________������ȷ��2λС����
��1�� Ũ��ˮ����ʯ�ң��������ƹ���ȣ� ��2�֣����������֣�
��2�������¶ȣ���߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��2�֣�
��3�� ͨ���۲����ݣ�����NH3��CO2ͨ����� ��1�֣�
��4������ c����1�֣���2�֣�
��5����ֹ���������ն��ఱ������ֹ������ˮ�������뷴Ӧ��ʹ���������ˮ�⣨��1�֣���3�֣�
��6��0.80��80%����Ч����û�п��ǿ�1�֣� ��2�֣�
���������������1�����ڰ�ˮ�д���ƽ���ϵNH3��H2O NH3��H2O
NH3��H2O NH4����OH��������Ҫ����Ũ��ˮ�Ʊ�����������Ũ��ˮ���뵽���������ƻ��������ƣ����ܽ�����з�������Һ��c(OH��)����ʹŨ��ˮ�ֽ����ɰ�����
NH4����OH��������Ҫ����Ũ��ˮ�Ʊ�����������Ũ��ˮ���뵽���������ƻ��������ƣ����ܽ�����з�������Һ��c(OH��)����ʹŨ��ˮ�ֽ����ɰ�����
��2����Ӧ2NH3��g��+CO2��g�� NH2COONH4��s��+Q�Ƿ��ȷ�Ӧ�������¶�ƽ��������Ӧ������У������ڰ���������������ɡ��Ұ�������������ֽ⣬���Է�Ӧ��������Ҫ�ñ�ˮ��ȴ��
NH2COONH4��s��+Q�Ƿ��ȷ�Ӧ�������¶�ƽ��������Ӧ������У������ڰ���������������ɡ��Ұ�������������ֽ⣬���Է�Ӧ��������Ҫ�ñ�ˮ��ȴ��
��3����Ϊ������뷴Ӧ�IJ����Ʒ�Ӧ���ʺ�����������Һ��ʯ������ƿ�������ǿ��Ʒ�Ӧ���г̶ȣ������������ٺ�ԭ���������ȣ���ͨ���۲����ݣ�����NH3��CO2ͨ�������
��4���Ʊ���������淋�װ����ͼ��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С���侧�����������Ȼ�̼�У������Ʒ��ʵ�鷽�����ù��˵õ�����������泥�NH2COONH4����һ�ְ�ɫ���壬�ֽ⡢���ܼ��Ⱥ�ɣ�Ӧ�����40�����º�ɣ����Դ�ѡc��
��5������������ˮ��������Ҫ�з�����װ�ã�˫ͨ�����ܵ������Ƿ�ֹҺ�嵹���������Ǽ������壬Ũ������ǿ�ᣬ�Ҿ�����ˮ�ԣ�����Ũ���������ն���İ�����ͬʱ��ֹ������ˮ�������뷴Ӧ��ʹ���������ˮ�⡣
��6��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7820g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000g����������̼��ƣ������ʵ���Ϊ1.000g��100g/mol��0.010mol������Ʒ�а�����������ʵ���Ϊx��̼��������ʵ���Ϊy������̼ԭ���غ�õ���x+y��0.01������Ϊ78x+79y��0.7820�����x��0.008mol��y��0.002mol������Ʒ�а�������淋����ʵ���������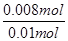 ��100%��80%��
��100%��80%��
���㣺���鰱���Ʊ�������ѡ��ʵ���������ơ����ʺϳ�ʵ�鷽������������Լ����ʺ����ļ����

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧͨ������ʵ��̽����Ӧԭ������֤���
ʵ��I����ɰֽ��ȥþ����������Ĥ���������ʢ�������з�̪�ı���̼��������Һ���ձ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz��ɫ���
��1����ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ����������
�²�2����ɫ���������ΪMgCO3
�²�3����ɫ���������Ϊ��ʽ̼��þ[yMg(OH)2?xMgCO3]
��2��Ϊ��ȷ������������¶���ʵ�飺
| ʵ����� | ʵ �� | ʵ������ | �� �� |
| ʵ��� | ��ʵ��I���ռ����������ȼ | ����ȼ�գ� ����ʵ���ɫ | ����ɷ�Ϊ �� �� |
| ʵ��� | ��ʵ��I�еİ�ɫ�������˳���ϴ�ӣ�ȡ������������ �� | �� | ��ɫ�������к���MgCO3 |
| ʵ��� | ȡʵ����е���Һ�������м����� ���� ���� ϡ��Һ | ������ɫ��������Һ��ɫ��dz | ��Һ�д���CO32- ���� |
ʵ�����ϴ�ӵIJ��������� ��
��3��Ϊ��һ��ȷ��ʵ��I�İ�ɫ������ijɷ֣��������¶���ʵ�飬װ����ͼ��ʾ��

��ȡ��������İ�ɫ������ 4.52 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ���װ��A����0.36 g��װ��B����1.76 g��
װ��C�������� ��
��ɫ������Ļ�ѧʽΪ���������������� ��
��4��д��þ�뱥��̼��������Һ��Ӧ�Ļ�ѧ����ʽ ��
Ϊ̽��Cl2��Ư�۵��Ʊ����й����ʣ�ij��ȤС����Ʋ�����������ʵ��̽������ش��������⣺
��1��ʵ������������װ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�H��_______��_______��_______��_______��_______�����й��ƿ���е��Լ�Ϊ_______��

��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ_______��
��3��ʵ������һƿ�ܷⲻ�ϵ�Ư����Ʒ�����п϶�����CaCl2�������ʵ�飬̽������Ʒ�г�CaCl2����е������������ʡ�
������������衣
����1����Ư��δ���ʣ�������Ca(ClO)2
����2����Ư��ȫ�����ʣ�������______��
����3����Ư�۲��ֱ��ʣ�������Ca(ClO)2��CaCO3��
�����ʵ�鷽��������ʵ�顣�����±���д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�õ�������ҩƷ���Թܡ��ιܡ������ܵĵ�����������ˮ������ˮ��Ʒ����Һ��1 mol��L-1 HCl��Һ�����Ƴ���ʯ��ˮ������ʾ�����ؼ���Ca2+��Cl-����
| | ʵ�鲽�� | Ԥ����������� |
| ����1 | ȡ��������Ư�����Թ��У��ȼ��� �ܽ���ٰ����ɵ�����ͨ�� �� | �� �������1������ �� �������2�����3������ |
| ����2 | ��ȷ��Ư�۱��ʣ�����ȡ��������Ư�����Թ��У��ȼ�������1 mol��L-1 HCl��Һ���ټ��� �� | �� �������2������ �� �������3������ |
ijʵ��С�����ͨп�̷ϸɵ���ڵĺ�ɫ�������̽����������·�����
��֪��I����ͨп�̵�صĺ�ɫ������Ҫ�ɷ�ΪMnO2��NH4Cl��ZnCl2�����ʡ�
II��������пΪ��ɫ��ĩ��������ˮ�������ᡢǿ����Һ�Ͱ�ˮ��
��ش��������⣺
��1���ڲ�����������___________��
��2��ijͬѧ������ҺA�ijɷֺ���NH4Cl��ZnCl2���������һ��ʵ�鷽������֤�������ȷ��Ҫ���ڴ���ϰ��±���ʽд��ʵ�������Ԥ������ͽ��ۡ�
��ѡ�Լ�������ˮ��2moL��L��1 HCI ��2 moL��L��1 HNO3 ��2 moL��L��1 NH3��H2O��6 moL��L��1 NaOH��0.1 moL��L��1 KSCN��0.1 moL��L��1 BaCl2��0.1 moL��L��1 AgNO3����ɫʯ����Һ����ɫʯ����ֽ
| ʵ����� | Ԥ������ | ���� |
| ����1����ȡ������ҺA��װa��b��c��֧�Թܣ���a�Թܣ�__ __________________________ | �а�ɫ�������� | ˵����ҺA����Cl�� |
| ����2����b�Թܣ�__________ __________________________ | ______________________ | _______________________ |
| ����3����c�Թܣ�__________ __________________________ | �Ȳ���_______________, ��____________________ | ˵����ҺA����Zn2+ |
��3��ȡ��������c�����Թܣ��μ���˫��ˮ���۲쵽�����������д���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
��4��Ϊ�ⶨ�ϸɵ���ж������̵�������������������ʵ�飺ȷ��ȡag��ǧ��ع��壬����ϡ���ᣬ����⻯����Һ����ַ�Ӧ����bmol/L��������Ʊ���Һ�ζ����õ�����ָʾ�����ζ����յ㣬�ظ�ʵ�飬ƽ��������������Ʊ���Һ�����ΪvmL����ϵ���ж������̵����������ļ������ʽΪ��________________________________��
���ζ��йط�Ӧ��MnO2+2I��+4H+=Mn2++I2+2H2O��I2+2S2O32��=2I��+S4O62����
����ʵ���ܴﵽ��ӦĿ�ĵ���
| A����ͼ��װ����ȡ���ռ����� | B����ͼ��װ����ȡ���ռ���ϩ |
| C����ͼ��װ�ý��������ճɻ� | D����ͼ��װ����ȡ�������� |


 2CuSO4+2H2O ) ���������������һ������
2CuSO4+2H2O ) ���������������һ������
