��Ŀ����
Ϊ�ⶨij�������������Fe��C����ĩ״��Ʒ����������������ij��ѧ�о���ѧϰС������йط�����������ʵ�顣

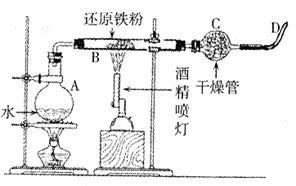
��1�������ͼ����ʾװ�ã�ʹ������Ʒ��ϡ���ᷴӦ�IJ���Ϊ ��
ʵ����������������е��������������Ϊ��״����������������Ʒ�����������������ⶨ�Ľ��ƫ�ͣ����ܵ�ԭ���� ��������ĸ��ţ�
A. ��Ӧ��������ȴ��δ�ٴε��������ܺ�ˮ����Һ����ƽ�� ����ȡ�������
B. ϡ�������
C. ˮ����������ˮ���
��2�������ͼ����ʾװ�ã���÷�Ӧǰ����й������������������Ʒ��������������Ϊ ������ͼ��װ���жϣ���ʵ���в���û��ʧ��ʵ�������� �����ƫ����ƫС���� ��ȷ����
��3����ȡ������ĩ5.72 g�������µ��������г�ַ�Ӧ���õ�CO2����224 mL(��״��)�����������ĩ������̼�����ʵ���֮��Ϊ ������ȡ���ݲ�ͬ������������ĩ���ֱ�ӵ�100 mL��ͬŨ�ȵ�H2SO4��Һ�У���ַ�Ӧ��õ�ʵ���������±���ʾ������ʵ�����������Һ�У������ܽ�������Ʒ������ ��

��1�������ͼ����ʾװ�ã�ʹ������Ʒ��ϡ���ᷴӦ�IJ���Ϊ ��
ʵ����������������е��������������Ϊ��״����������������Ʒ�����������������ⶨ�Ľ��ƫ�ͣ����ܵ�ԭ���� ��������ĸ��ţ�
A. ��Ӧ��������ȴ��δ�ٴε��������ܺ�ˮ����Һ����ƽ�� ����ȡ�������
B. ϡ�������
C. ˮ����������ˮ���
��2�������ͼ����ʾװ�ã���÷�Ӧǰ����й������������������Ʒ��������������Ϊ ������ͼ��װ���жϣ���ʵ���в���û��ʧ��ʵ�������� �����ƫ����ƫС���� ��ȷ����
| ��Ӧǰ������װ��+ ϡ��������/g | ��Ӧǰ�� ������Ʒ����/g | ��Ӧ������װ��+ ��ƿ��ʣ���������/g |
| a | m | b |
| ʵ����� | �� | �� | �� |
| ����������Ʒ������/g | 1.43 | 2.86 | 8.58 |
| ������������/L����״���� | 0.56 | 1.12 | 2.24 |
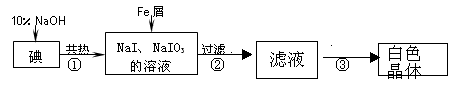
��10�֣���1����Y����б��ʹ������Һ���뵽������Ʒ�С�A����1�֣�2�֣�
��2�� ��ƫ��(H2Я��ˮ�����ݳ�)����2�֣�4�֣�
��ƫ��(H2Я��ˮ�����ݳ�)����2�֣�4�֣�
��3��10:1 ��2.86 g������2�֣�4�֣�
��2��
 ��ƫ��(H2Я��ˮ�����ݳ�)����2�֣�4�֣�
��ƫ��(H2Я��ˮ�����ݳ�)����2�֣�4�֣� ��3��10:1 ��2.86 g������2�֣�4�֣�
�����������1��Ҫʹ����Ʒ��ϡ���ᷴӦ���͵�ʹ����Ʒ��ϡ����Ӵ����ʲ���Ϊ��Y����б��ʹ������Һ���뵽������Ʒ�С��������ƫ�͵�ԭ��Aѡ���бȽϺ������ʴ�A��
��2�����ݷ�Ӧ���������Һ����=�μӷ�Ӧ��������+�������ϡ���������-��Ӧ�ų�����������������������Ʒ��������������Ϊ
 ����ΪH2��Я��ˮ�����ݳ�����ʵ��������ƫ��
����ΪH2��Я��ˮ�����ݳ�����ʵ��������ƫ����3�������µ��������г�ַ�Ӧ��Fe��C��������������Ӧ������CO2����224 mL(��״��)����0.01mol�����ݻ�ѧ����ʽ�����C�����ʵ���Ϊ0.01mol������Ϊ0.12g��������������Ϊ5.72g-0.12g=5.6g�����ʵ���Ϊ0.1mol����������ĩ������̼�����ʵ���֮��Ϊ0.1mol:0.01mol=10:1��
���ݱ������ݼ��㣬ʵ�����������ĩ�ǹ����ģ��ᷴӦ�꣬������������������2.24L���㣬������ʵ���Ϊ0.1mol, ʵ��������ǹ����ģ����ݸ�����������������1.12L���㣬�ᷴӦ��0.05mol��ʣ��0.05mol����ʵ�����������Һ�У������ܽ�������Ʒ������2.86g��
������������Ҫ�����йػ�ѧ����ʽ�ļ���ͻ�ѧʽ�ļ��㣬�ѶȽϴ��������غ㶨�ɣ���Ӧ���������Һ����=�μӷ�Ӧ��������+�������ϡ���������-��Ӧ�ų�������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


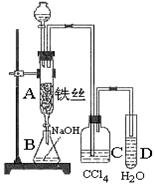
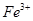 ����ܺ���
����ܺ���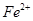 ����Ҫȷ�����е�
����Ҫȷ�����е� ��Һ
��Һ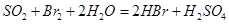 ��Ȼ���������
��Ȼ��������� ��Һ�����ʵ�������ø������2��33g�����ڴ���֪����Y��
��Һ�����ʵ�������ø������2��33g�����ڴ���֪����Y��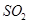 ���������Ϊ ��
���������Ϊ �� ��Q���塣Ϊ�����������̽��ʵ��״�ã�ͼ�мг�����ʡ�ԣ���
��Q���塣Ϊ�����������̽��ʵ��״�ã�ͼ�мг�����ʡ�ԣ���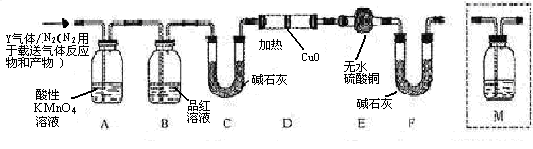

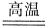 2Fe+3CO
2Fe+3CO ���˷�Ӧ�����ڹ�ҵ��ұ������������Ӧ�У���Ϊ��������������________���ѧʽ�����ڸ÷�Ӧ�У���������1 mol
���˷�Ӧ�����ڹ�ҵ��ұ������������Ӧ�У���Ϊ��������������________���ѧʽ�����ڸ÷�Ӧ�У���������1 mol  ����ת����_____mol���ӣ����ɵ�CO
����ת����_____mol���ӣ����ɵ�CO