��Ŀ����
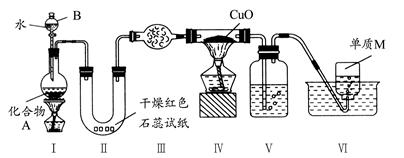
��ҵ������м��ԭ���Ʊ��⻯�Ƶ���Ҫ��������ͼ��
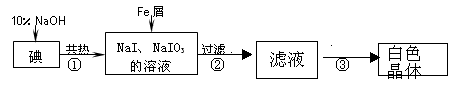
��1����Ԫ��λ�����ڱ��е� ���ڣ��� �壻
��2����Ӧ�ٵĻ�ѧ����ʽ ��
��3���жϷ�Ӧ���е��Ƿ�����ȫ��Ӧ�IJ����� ��
��4������Һ�ڵ����·����NaI��Ʒ�Ĺ����У�Ҫ��ֹNaI����������ȡ�Ĵ�ʩ����� ��
��5��ijͬ�Ʋⲽ����еõ��İ�ɫ������NaI��NaIO3��NaOH�Ļ���������·������м��飬ʵ�����������Ʋ���ȷ��
��֪��IO3-ʮ5I-+6H+=3I2+3H2O��NaIO3ˮ��Һ�����ԡ�
��ѡ�Լ���lmol��LH2SO4��2mol��LHNO3��������Һ����̪��Һ��ʯ����Һ������ˮ��������������Ʒ��ѡ��
Ҫ�õ�������NaI�������һ���IJ����� ����������ƣ�

��1����Ԫ��λ�����ڱ��е� ���ڣ��� �壻
��2����Ӧ�ٵĻ�ѧ����ʽ ��
��3���жϷ�Ӧ���е��Ƿ�����ȫ��Ӧ�IJ����� ��
��4������Һ�ڵ����·����NaI��Ʒ�Ĺ����У�Ҫ��ֹNaI����������ȡ�Ĵ�ʩ����� ��
��5��ijͬ�Ʋⲽ����еõ��İ�ɫ������NaI��NaIO3��NaOH�Ļ���������·������м��飬ʵ�����������Ʋ���ȷ��
��֪��IO3-ʮ5I-+6H+=3I2+3H2O��NaIO3ˮ��Һ�����ԡ�
��ѡ�Լ���lmol��LH2SO4��2mol��LHNO3��������Һ����̪��Һ��ʯ����Һ������ˮ��������������Ʒ��ѡ��
| ʵ�鷽�� | ʵ������ | ���� |
| ����ɫ��������ˮ������2�ε�����Һ | �õ���ɫ��Һ |  |
| ȡ������ҺҺ���Թ�A�У� . | ��Һ����ɫ | ��Һ�к�IO3һ |
| ��ȡ������ҺҺ���Թ�B�У� . | . | ��Һ�к�����0Hһ |
Ҫ�õ�������NaI�������һ���IJ����� ����������ƣ�
��16�֣�
��1�� �壨1�֣�����A��1�֣���
��2��3I2+6NaOH 5NaI+NaIO3+3H2O ��2�֣���
5NaI+NaIO3+3H2O ��2�֣���
��3��ȡ������ӦҺ���Թ��У����Թܼ��뼸�ε�����Һ������Һ������˵����δ��Ӧ�꣬��������˵������ȫ��Ӧ��2�֣���
��4������������2�֣���
��5������������1 mol/LH2SO4 ��2�֣������뼸�η�̪��Һ��2�֣�����Һ��죨2�֣����ؽᾧ. ��2�֣�
��1�� �壨1�֣�����A��1�֣���
��2��3I2+6NaOH
 5NaI+NaIO3+3H2O ��2�֣���
5NaI+NaIO3+3H2O ��2�֣�����3��ȡ������ӦҺ���Թ��У����Թܼ��뼸�ε�����Һ������Һ������˵����δ��Ӧ�꣬��������˵������ȫ��Ӧ��2�֣���
��4������������2�֣���
��5������������1 mol/LH2SO4 ��2�֣������뼸�η�̪��Һ��2�֣�����Һ��죨2�֣����ؽᾧ. ��2�֣�
���������
��1��������±��Ԫ�أ�λ�ڵ������ڵ�VIIA�壻
��2���������ͼ��֪������NaI��NaIO3��������ԭ��Ӧ�Ĺ��ɿ���ƽ���绯��Ӧ��������ʾ��
��3���ж�I2�Ƿ�Ӧ����Ҫ����I2���������λ��ȡ������ӦҺ���Թ��У����Թܼ��뼸�ε�����Һ������Һ������˵����δ��Ӧ�꣬��������˵������ȫ��Ӧ��
��4��I-�ױ������е�O2������Ϊ��ֹ�����������������Ϻã�
��5�����������Ϣ��֪������IO3-ʱ��Ӧ�ü����ᣬ�����ܼ����ᣬ��Ϊ���������I-ΪI2���ż���IO3-��HIO3��ǿ�ᣬNaIO3��ˮ�⣬��HIҲ��ǿ�ᣬNaIҲ��ˮ�⣬��Һ��OH-��������ˮ���������ֱ�������ָʾ��������OH-���ڣ�Ҫȥ��NaI��������NaIO3������NaI��NaIO3���ܽ�����¶ȱ仯��ͬ���ﵽ�����ᴿ��Ŀ�ĵķ������ؽᾧ���ٽᾧ����2�ļ��顢����ѡ��������������IO3-��OH-�������ᴿ�ķ���ѡ��

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
�����Ŀ
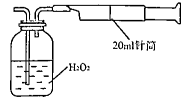 ��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��
��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��