��Ŀ����
�Ѱۣ���Ҫ�ɷ���TiO2�����㷺�������ᡢ���ϡ���ֽ����ҵ�����������Ҵ���ˮ������Ĵ�������ͼ������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ�������Ѱ۲���ø���ƷFeSO4��7H2O�Ĺ�������ͼ��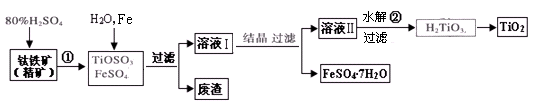
��1�������������ᷢ����Ӧ�ٵĻ�ѧ����ʽΪ ����TiOSO4��FeSO4��Һ�м���Fe��Ŀ���� ��
��2����Һ����TiOSO4�ڼ��������·���ˮ�ⷴӦ�ڵ����ӷ���ʽΪ ���ɻ������õ������� ��
��3��Ϊ�ⶨ��Һ����TiOSO4�ĺ���������ȡ������Һ10 mL��ˮϡ����100 mL���ӹ������ۣ������ʹ����ȫ��Ӧ��3TiO2+ +Al+6H+=3Ti3++Al3++3H2O�����˺�ȡ����Һ20.00 mL�������еμ�2��3��KSCN��Һ��ָʾ������ ����һ�ֲ������������ƣ��μ�0.1000mol��L��1 FeCl3��Һ������Һ���ֺ�ɫ�ﵽ�ζ��յ㣬��ȥ��30.00mL FeC13��Һ��������Һ��TiOSO4�����ʵ���Ũ���� ��
��12�֣���1��FeTiO3+2H2SO4=TiOSO4+FeSO4+2H2O��2�֣�
��ֹFe2�����������Ի�ô����ĸ���ƷFeSO4��7H2O��2�֣�
��2��TiO2+ +2H2O=H2TiO3�� +2H+��2�֣� H2SO4��2�֣�
��3����ʽ�ζ��ܣ�2�֣� 1.50 mol?L��1��2�֣�
���������������1�����������غ㶨�ɼ������֪����Ϊ���ֽⷴӦ����Ӧ����FeTiO3��H2SO4��������TiOSO4��FeSO4��H2O���ù۲취����ƽ����������FeSO4��7H2O��Ŀ���жϣ�����Fe��ʹFe3+��ԭΪFe2+������Fe2+�����������Ի�ô����ĸ���Ʒ�̷�����2��������ǿ�ᣬ��SO42������ˮ�⣬��H2TiO3�����ᣬTiO22+��ˮ�⣬������ʹTiOSO4����ˮ�⣬����H2TiO3������H2SO4�����ݵ�ɡ�ԭ���غ����д�����ӷ���ʽ���÷�Ӧ���ɵ�H2SO4�����ڷ�Ӧ����ѭ�����ã���˿ɻ��գ���3��FeCl3��Һ�����ԣ���Ҫʹ����ʽ�ζ���ʢ�ţ�3TiO2++Al+6H+=3Ti3++Al3++3H2O��Ti3+(��ɫ) +Fe3++H2O = TiO2+(��ɫ) +Fe2++2H+��Fe3++3SCN�� Fe(SCN)3��Ѫ��ɫ������ﵽ�ζ��յ�ʱ���ĵ�FeCl3��Fe3+Ϊ0.1000mol��L��1��30.00��10��3L����Fe3+��Ӧ��Ti3+����Al��ԭ��TiO2+��Fe3+�����ʵ�����ȣ�����0.1000mol��L��1��30.00��10��3L��10mL������Һ��TiOSO4Ϊ0.1000mol��L��1��30.00��10��3L��
Fe(SCN)3��Ѫ��ɫ������ﵽ�ζ��յ�ʱ���ĵ�FeCl3��Fe3+Ϊ0.1000mol��L��1��30.00��10��3L����Fe3+��Ӧ��Ti3+����Al��ԭ��TiO2+��Fe3+�����ʵ�����ȣ�����0.1000mol��L��1��30.00��10��3L��10mL������Һ��TiOSO4Ϊ0.1000mol��L��1��30.00��10��3L��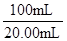 ��
��
��c(TiOSO4)=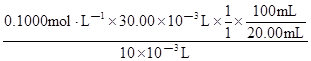 =1.50 mol��L��1��
=1.50 mol��L��1��
���㣺�������Ʊ����������⣬�ǽ���߿����ȵ㣬��Ҫ�����˺��ķ�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ����д������Ͷ�ϵ�Ŀ�ġ�ԭ�Ӿ��á��ζ��ܵ�ѡ�ζ�ʵ�����ݴ��������ȡ�

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�ʵ����������ij��ӻ�β�����ռ���ѡ��װ����ȷ���� 
| A������Cl2 | B������HCl | C������H2 | D������NH3 |
��11�֣�������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᡣ��Ӧԭ����
ʵ�鷽����һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
��֪����������Է���������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g�����������л���һ�㶼�й̶��۵㡣
��1��������Ϊ ��������Ϊ ��
��2����ɫҺ��A�� �����Լ���A���Լ��� �������� ��
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۡ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ�����ڴ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У����ȣ��ܽ⣬�� | �õ���ɫ�������ɫ��Һ | �������� |
| �� | ȡ������Һ���Թ��У� �� | ���ɰ�ɫ���� | ��Һ����Cl- |
| �� | �����ɫ���壬 �� | �� | ��ɫ�����DZ����� |
��4�����Ȳⶨ����ȡ1.220g��Ʒ�����100ml�״���Һ����ȡ25.00ml��Һ���ζ�������KOH�����ʵ���Ϊ2.40��10-3mol����Ʒ�б�������������Ϊ ��������λ��Ч���֣���
��14�֣���ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ���Ҫ�ɷ�ΪCuFeS2����������ʯ��Ϊ�ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
�õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��1/10������ƿ�У���0.05mol/L������Һ���еζ������ı�����Һ20.00mL����ش��������⣺
��1������Ʒ��ϸ���ٽ��з�Ӧ����Ŀ���� ��������ҺӦʢ���ڣ����ʽ������ʽ���� �ζ����С�
��2��װ��a��������
������ţ���
| A����ȥ�����еĶ�����̼ | B����ȥ�����е�ˮ���� |
| C�������������� | D�������ڹ۲졢���ƿ������� |
��4��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ���� ��
��5��ͨ�������֪���û�ͭ��Ĵ���Ϊ ��