��Ŀ����
��14�֣���ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ���Ҫ�ɷ�ΪCuFeS2����������ʯ��Ϊ�ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
�õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��1/10������ƿ�У���0.05mol/L������Һ���еζ������ı�����Һ20.00mL����ش��������⣺
��1������Ʒ��ϸ���ٽ��з�Ӧ����Ŀ���� ��������ҺӦʢ���ڣ����ʽ������ʽ���� �ζ����С�
��2��װ��a��������
������ţ���
| A����ȥ�����еĶ�����̼ | B����ȥ�����е�ˮ���� |
| C�������������� | D�������ڹ۲졢���ƿ������� |
��4��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ���� ��
��5��ͨ�������֪���û�ͭ��Ĵ���Ϊ ��
��14�֣�
��1��ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ�2�֣���ʽ��2�֣�
��2��B D��2�֣�
��3��ƫ�ͣ�2�֣� 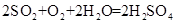 ��2�֣�
��2�֣�
��4��ʹ��Ӧ���ɵ�����ȫ������dװ���У�ʹ�ⶨ�����ȷ��2�֣� ��5��80%��2�֣�
���������������1������Ʒ��ϸ���ٷ�Ӧ�����������ı������Ŀ����ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ���ҺΪI2��Һ�����������ԣ��������ܣ������ü�ʽ�ζ���ʢ�ţ�Ӧѡ����ʽ�ζ��ܡ�
��2��װ��a�е�Ũ����������տ����е�ˮ������ͬʱ����ð�������ݵ����������������ͨ�������ʴ�Ϊ��BD��
��3��ȥ��cװ�ã������ж���������ˮ��Һ�л��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2SO2+O2+H2O=2H2SO4���ⶨ���ƫ�͡�
��4����ͭ�����ȷֽ����ɶ��������һϵ�в���ֽ���Ϻ���Ȼͨ����������Խ������Ķ�������ȫ���ų�ȥ������dװ���У�ʹ�����ȷ��
��5������������ԭ��Ӧ�е�ʧ����������Ⱥ�SԪ���غ�ɵõ���2I2��2SO2��CuFeS2�����ĵ�0.05mol/L������Һ20.00mLʱ�������ĵĵⵥ�ʵ���Ϊ��0.05mol/L��0.02L=0.0010mol�����Ի�ͭ��������ǣ�0.5��0.0010mol��184g/mol��10=0.92g�������䴿���ǣ�0.92g��1.15g��100%=80%��
���㣺���⿼������������ζ�ԭ����ʵ�鷽����������������ѧ���㡣

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�(14��)������һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᡣijѧϰС���ͬѧ���Ը�����Ϊԭ����ˮ�⡪������ˮ��ѭ��������ȡ���ᡣ
|
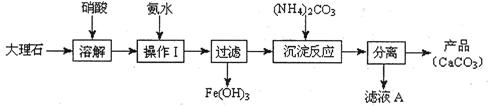
 �����������Ϣ�ش��������⣺
�����������Ϣ�ش��������⣺��1��ͼʾ�٢ڵ�������ˮ�����������ͼ1��װ���н��еģ�ָ��װ��A������ ��
��2��ͼʾ�٢ڵ�������ˮ������У���������������Ӧ��ʱ�����������ͬ������£��ı䷴Ӧ�¶��Կ��췴Ӧ�¶ȶԲ������ʵ�Ӱ�죬�������ͼ2��ʾ����ѡ����ѵķ�Ӧ�¶�Ϊ ��Ϊ�˴ﵽͼ2��ʾ���¶ȣ�ѡ��ͼ1��ˮԡ���ȣ����ŵ��� ��
��3����ͼʾ�ۢ��еIJ����漰�����ˣ�ϴ�ӡ���������й�˵����ȷ���� ��
A.��ʵ������У�ͨ��������ȴ������Һ�����Եõ��ϴ�ľ�����������ڳ���
B.��ϴ�ӳ���ʱ��Ӧ��Сˮ��ͷ��ʹϴ�Ӽ�����ͨ��������
C.Ϊ�˼���ϴ���Ƿ���ȫ��Ӧ��������ƿ�밲ȫƿ֮����Ƥ�ܣ�������ƿ�Ͽڵ���������Һ���Թ��н������ʵ�顣
D.Ϊ�˵õ�����ľ��壬����ѡ����������ֱ�Ӽ��ȣ����ڸ���������ȴ��
��4��Ҫ�ⶨ���ᾧ�壨H2C2O4��2H2O���Ĵ��ȣ���ȡ7.200g�Ʊ��IJ��ᾧ����������ˮ���250mL��Һ��ȡ25.00mL������Һ����ƿ�У���0.1000mol/L���Ը��������Һ�ζ�
��5H2C2O4+2MnO4��+6H+��2Mn2++10CO2��+8H2O����
��ȡ25.00mL������Һ�������� ��
���ڲ��ᴿ�Ȳⶨ��ʵ������У�����˵����ȷ���ǣ� ��
A.��ϴ�ζ���ʱ��Ӧ�ӵζ����Ͽڼ������������Һ��ʹ�ζ����ڱڳ����ϴ
B.��Һ��ȡ������Һʱ���轫���촦��Һ�崵����ƿ����ʹʵ�����ƫ��
C.�ζ�ʱ�������������ڿ�ס���������Ŀ���������������ʹ���ɶ�©����Һ
D.�ζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ʹʵ�����ƫ��
���жϵζ��Ѿ��ﵽ�յ�ķ����ǣ� ��
�ܴﵽ�ζ��յ�ʱ�����ĸ��������Һ��20.00mL������ᾧ��Ĵ���Ϊ ��
 2CaO+2S02��+O2����
2CaO+2S02��+O2����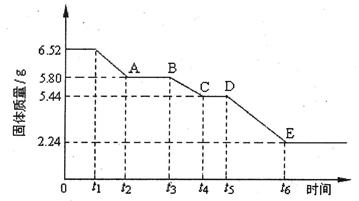
 ��Na2Cr2O7��4H2SO4�D��
��Na2Cr2O7��4H2SO4�D�� ��Na2SO4��Cr2(SO4)3��5H2O
��Na2SO4��Cr2(SO4)3��5H2O
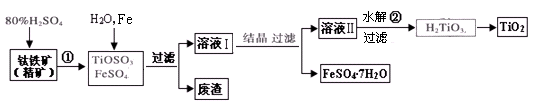
 �ۣ���Ԫ����+2��)��ʵ�������û�ͭ��Ϊԭ����ȡ����ͭ������(Fe2O3)���������£�
�ۣ���Ԫ����+2��)��ʵ�������û�ͭ��Ϊԭ����ȡ����ͭ������(Fe2O3)���������£�

 =
=




 +CaS+BaCl2�������Ǵӱ��պ�Ĺ����з���õ��Ȼ��������ʵ���������(��֪�Ʋ�����ˮ������������)�����ڿո��������д�������ơ�
+CaS+BaCl2�������Ǵӱ��պ�Ĺ����з���õ��Ȼ��������ʵ���������(��֪�Ʋ�����ˮ������������)�����ڿո��������д�������ơ�
