��Ŀ����
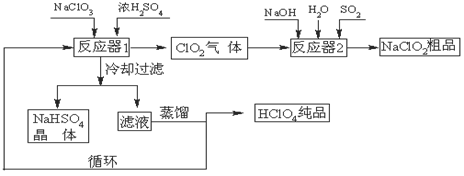
���������Ҫ�ɷ�ΪFeTiO3���ɱ�ʾΪFeO��TiO2������������MgO��CaO��SiO2�����ʡ������������Ʊ�����ӵ�ص缫���ϣ������Li4Ti5O12�����������LiFePO4���Ĺ�ҵ��������ͼ��ʾ�� 
��֪��FeTiO3�����ᷴӦ�����ӷ���ʽΪ��FeTiO3��4H+��4Cl-��Fe2+��TiOCl42-��2H2O
��1��������FeTiO3����Ԫ�صĻ��ϼ��� ��
��2������A�ijɷ��� ��
��3����ҺB��TiOCl42- ת������TiO2�����ӷ���ʽ�� ��
��4����Ӧ���й���TiO2ת����(NH4)2Ti5O15��Һʱ��TiԪ�صĽ������뷴Ӧ�¶ȵĹ�ϵ����ͼ��ʾ����Ӧ�¶ȹ���ʱ��TiԪ�ؽ������½���ԭ���� ��
��5����Ӧ�۵Ļ�ѧ����ʽ�� ��
��6������ҺD�Ʊ�LiFePO4�Ĺ����У�����17%˫��ˮ��H2C2O4���������� ��
��7������������ﮣ�Li4Ti5O12������������ﮣ�LiFePO4�����缫��ɵ�أ��乤��ԭ��Ϊ��Li4Ti5O12��3LiFePO4 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4
�õ�س��ʱ������Ӧʽ�� ��
��12�֣�ÿ��2�֣�
��1��+2
��2��SiO2
��3��TiOCl42-��H2O  TiO2����2H+��4Cl-
TiO2����2H+��4Cl-
��4���¶ȹ���ʱ����Ӧ�ﰱˮ����˫��ˮ�������ֽ�
��5�� (NH4)2Ti5O15��2LiOH��Li2Ti5O15����2NH3��H2O����2NH3��2H2O��
��6��20/9��1�֣�
��7��LiFePO4 �C e-��FePO4��Li+��1�֣�
���������������1��������Ŀ������Ϣ���ɱ�ʾΪFeO��TiO2����֪Fe�Ļ��ϼ�Ϊ��+2��
��2��FeTiO3��MgO��CaO��HCl��Ӧ��ʣ�µĹ���ֻ��SiO2��
��3�����˺�δ��������Ӧ�����TiOCl42-��H2O��Ӧ������TiO2��ͬʱ����H+��Cl?��
��4��TiOCl42- ת��ΪTiO2ʱ����Ҫ���뷴Ӧ��˫��ˮ����ˮ�����������������ֽ⡣
��5��(NH4)2Ti5O15Ϊ��Σ�LiOHΪǿ��������ֽⷴӦ�����ݷ�Ӧ���ɣ�����д����ѧ����ʽ��
��6��������Ӧ���̣�H2O2��Fe2+����ΪFe3+��H2C2O4��Fe3+��ԭΪFe2+��H2O2��O��-1�۱�Ϊ-2�ۣ�H2C2O4��+3�۱�Ϊ+4�ۣ����ݵ���ת��������ȵã�m(H2O2)��17��200g/mol��2=m(H2C2O4)��90g/mol��2����m(H2O2)��m(H2C2O4)= 20/9��
��7����س��ʱ������ӦΪʧ���ӷ�Ӧ��LiFePO4��Feʧȥ1�����ӣ���+2�۱�Ϊ+3�ۣ�����FePO4��ͬʱ�õ�Li+��
���㣺���⿼�黯ѧ��������ͼ�ķ��������ϼ۵��жϡ���ѧ����ʽ�����ӷ���ʽ����д���й�������ԭ��Ӧ�ļ��㡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����й��ڻ�ѧ���������������ʶ�������
| A�������÷���֬�Ʒ��� |
| B��ˮ��������������ľ�ķ���� |
| C��ú��������Һ���������仯�ɱ�Ϊ���ȼ�� |
| D���������ƳɵIJ۳������ܷ�����Ũ�����Ũ���� |
��ҵ���ð���ʯ�Ʊ��ߴ�����þ�Ĺ����������£�
��֪����ʯ����Ҫ�ɷֿɱ�ʾΪ��
CaO 32.50%��MgO 20.58%��Fe2O3 2.18%��SiO2 0.96%������ 43.78%
��1��Ϊ����߰���ʯ������Ч�������Բ�ȡ�Ĵ�ʩ�� ��
����ʵ���������հ���ʯ����Ҫ���������ƾ��ơ����ż����⣬����Ҫ ��
| A�������� | B������ | C�������� | D��ʯ���� |

��3������Һ�л��CaSO4��2H2O�IJ���������Ũ���� �����ˡ�ϴ�ӡ����
��4��д��������Ӧ�е����ӷ���ʽ�� ����������Ƿ�ϴ���ķ����ǣ� ��
��5��������Ӧ�����У��¶ȶԲ�Ʒ�Ĵ���Ҳ��һ����Ӱ�졣��ͼΪ��Ӧ�¶ȶ�����þ���ȵ�Ӱ�졣����ȷ������Ӧ�������¶�Ϊ ��

ij�سィ�еġ���̬ũҵ�Ƽ�������������ũҵ���¼���ʾ�����ƹ���أ�Ҳ����һ���۹����е���̬ũҵ������һЩ����˼·������Ϊ�������� (����)��
| A����ũ�ҷ��뻯���ۺ�ʹ�ã����������Ч�� |
| B���Դ����е�ֲ��ʩ��������CO2���Դٽ��������� |
| C����ֲ����ֳ�����������ϣ��ȿɸ��ƻ����ֿ����ũ����ҵ�IJ��� |
| D��������狀���ʯ�һ��ʹ�ã��ڸ������ṩӪ��Ԫ�ص�ͬʱ�����ܽ������������� |
���л�ѧ��ҵ�й��豸��ԭ�ϡ���Ӧ���������� ��������
| A�������Ƽ�ƴ����������̼������ʳ�Ρ�������̼������30 �桫35 ��İ�������ʳ��ˮ����CO2 |
| B���Ӵ������������¯��������V2O5��4000��5000 �� |
| C���ϳɰ�������¯����̿������ý��500 �� |
| D����������������ϳ���������������Ͻ�8000 �� |


 Al(OH)3+3H+��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���
Al(OH)3+3H+��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���