��Ŀ����
15�֣���ֽͨ�ŵijɷ�����ά�أ������ڵ�ֽ�������У�������ֽ����Ϳ�������Ĺ��գ�����������ף���ֹī����ɢ����ش��������⣺
��1�����Ƿ���ֽ�Żᷢ�����Ը�ʴ����ࡢ����������вֽ������ı��档���������飬�������Ը�ʴ��Ҫ����ֽ��Ϳ�������Ĺ����йأ����еĻ�ѧԭ����______��Ϊ��ֹֽ�ŵ����Ը�ʴ������ֽ���м���̼��Ƶ����Ӽ����ù���ԭ���Ļ�ѧ�����ӣ�����ʽΪ______��
��2��Ϊ�˱�����Щֽ��������˽����ȡ���д�ʩ��
������������Һ����ϡ����������Һ��ˮ�ȣ�����������������Ҫ������______��
������Zn(C2H5)2��Zn(C2H5)2������ˮ��Ӧ��������п�����顣�û�ѧ�����ӣ�����ʽ��ʾ�÷�����������п����ֹ���Ը�ʴ��ԭ��______��
��3���ִ���ֽ���ճ����Ѱۣ�TiO2������������Ѱ۵�һ�ֹ�ҵ�Ʒ�������������Ҫ�ɷ֣�FeTiO3��Ϊԭ�ϰ��¹��̽��еģ���������л�ѧ����ʽ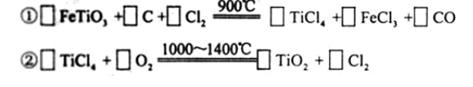
��1�����Ƿ���ֽ�Żᷢ�����Ը�ʴ����ࡢ����������вֽ������ı��档���������飬�������Ը�ʴ��Ҫ����ֽ��Ϳ�������Ĺ����йأ����еĻ�ѧԭ����______��Ϊ��ֹֽ�ŵ����Ը�ʴ������ֽ���м���̼��Ƶ����Ӽ����ù���ԭ���Ļ�ѧ�����ӣ�����ʽΪ______��
��2��Ϊ�˱�����Щֽ��������˽����ȡ���д�ʩ��
������������Һ����ϡ����������Һ��ˮ�ȣ�����������������Ҫ������______��
������Zn(C2H5)2��Zn(C2H5)2������ˮ��Ӧ��������п�����顣�û�ѧ�����ӣ�����ʽ��ʾ�÷�����������п����ֹ���Ը�ʴ��ԭ��______��
��3���ִ���ֽ���ճ����Ѱۣ�TiO2������������Ѱ۵�һ�ֹ�ҵ�Ʒ�������������Ҫ�ɷ֣�FeTiO3��Ϊԭ�ϰ��¹��̽��еģ���������л�ѧ����ʽ

��1������ˮ��������Ի�������������������ά��ˮ�⣬ʹ�߷��������ѣ�CaCO3��2H��=Ca2����H2O��CO2����
��2���ٹ����ļ�ͬ�����ܻᵼ����ά��ˮ�⣬����鼮����
��Zn(C2H5)2+ H2O��ZnO��2C2H6����ZnO��2H��=Zn2����H2O��
��3��2 6 7 2 2 6�� 1 1 1 2
��2���ٹ����ļ�ͬ�����ܻᵼ����ά��ˮ�⣬����鼮����
��Zn(C2H5)2+ H2O��ZnO��2C2H6����ZnO��2H��=Zn2����H2O��
��3��2 6 7 2 2 6�� 1 1 1 2
��1����Ϊ����ˮ��������Ի�������������������ά��ˮ�⣬ʹ�߷��������ѡ�̼������������ɵ������ӣ��ӽ������ԣ�����ʽΪCaCO3��2H��=Ca2����H2O��CO2����
��2��������ά���ڽ���������Ҳ��ˮ�⣬�Ӷ�����鼮���𣻸��ݷ�Ӧ�����������γɷ���ʽΪZn(C2H5)2+ H2O��ZnO��2C2H6������������п�������������ӣ����������ԣ�����ʽΪZnO��2H��=Zn2����H2O��
��3�����ݵ�ʧ�����غ������ƽ����Ӧ������ʧȥ1�����ӣ�̼ʧȥ2�����ӣ������õ�2�����ӡ���Ӧ���������õ�4�����ӣ���ԭ��ʧȥ1�����ӡ�
��2��������ά���ڽ���������Ҳ��ˮ�⣬�Ӷ�����鼮���𣻸��ݷ�Ӧ�����������γɷ���ʽΪZn(C2H5)2+ H2O��ZnO��2C2H6������������п�������������ӣ����������ԣ�����ʽΪZnO��2H��=Zn2����H2O��
��3�����ݵ�ʧ�����غ������ƽ����Ӧ������ʧȥ1�����ӣ�̼ʧȥ2�����ӣ������õ�2�����ӡ���Ӧ���������õ�4�����ӣ���ԭ��ʧȥ1�����ӡ�

��ϰ��ϵ�д�
�����Ŀ
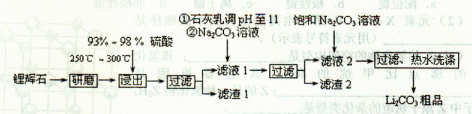
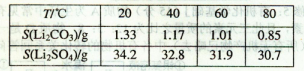
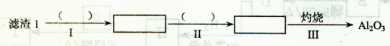
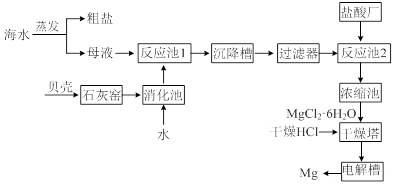
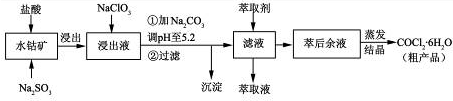
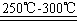 Li2SO4+Al2O3��H2O��
Li2SO4+Al2O3��H2O��