��Ŀ����
����Ŀ����ͼ��ʾ��һ���绯ѧװ�õ�ʾ��ͼ��
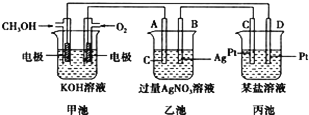
��ش��������⣺
��1��ͼ�б�����__(����ԭ���������������������Ƴ���)��
��2��B�缫��������__(����������������������������������������)��
��3��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ��___��
��4���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ___��
��5�����ҳ���B(Ag)����������10.8gʱ���׳�������������O2�����Ϊ___L(��״��)����ʱ������ij�缫����2.8gij������������е�ij����Һ������___(����ĸ)��
A.MgSO4 B.CuSO4 C.NaCl D.AgNO3.
���𰸡����� ���� CH3OH-6e-+8OH-�T6H2O+CO32- 4AgNO3+2H2O![]() 4Ag+O2��+4HNO3 0.56 BD
4Ag+O2��+4HNO3 0.56 BD
��������
��1��ͼ�м׳����Է�����������ԭ��Ӧ������ԭ��أ�ͨȼ�ϵĵ缫�Ǹ�����ͨ�����ĵ缫��������
��2��ͨ��״��ĵ缫Ϊ������ͨ�������ĵ缫Ϊ����������������ԭ��ظ����ĵ缫Ϊ����������ԭ��������ĵ缫Ϊ������
��3���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ��
��4���ҳ���A�缫�����������ӷŵ硢�����������ӷŵ磬�ݴ���д�ҳ��з�Ӧ�Ļ�ѧ����ʽ��
��5������ת�Ƶ�����Ƚ�ϵ缫��Ӧʽ���м��㡣
��1��ͼ�м׳����Է�����������ԭ��Ӧ������ѧ��ת��Ϊ���ܣ�����ԭ��أ��ҳغͱ������ڵ��أ������������缫һ�������ǵ�Ƴأ�
�ʴ�Ϊ�����أ�
��2��ͨ��״��ĵ缫Ϊ������ͨ�������ĵ缫Ϊ������A����ԭ���������Ϊ����������B��������
�ʴ�Ϊ��������
��3���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ���缫��ӦʽΪCH3OH-6e-+8OH-�T6H2O+CO32-��
�ʴ�Ϊ��CH3OH-6e-+8OH-�T6H2O+CO32-��
��4���ҳ���A�缫�����������ӷŵ硢�����������ӷŵ磬�����ҳص�ط�ӦʽΪ4AgNO3+2H2O![]() 4Ag+O2��+4HNO3��
4Ag+O2��+4HNO3��
�ʴ�Ϊ��4AgNO3+2H2O![]() 4Ag+O2��+4HNO3��
4Ag+O2��+4HNO3��
��5��B(Ag)������������10.8gʱ������Ag++e- =Ag ��n(Ag)=![]() =0.1mol����ת�Ƶ���Ϊ0.1mol������ת�Ƶ�����ȣ��׳���1molO2ת��Ϊ������ת��4mol���ӣ���ת�Ƶ���Ϊ0.1mol����������O2����µ����=
=0.1mol����ת�Ƶ���Ϊ0.1mol������ת�Ƶ�����ȣ��׳���1molO2ת��Ϊ������ת��4mol���ӣ���ת�Ƶ���Ϊ0.1mol����������O2����µ����=![]() ��22.4L/mol=0.56L����ʱ������ij�缫����2.8gij������ת�Ƶ���Ϊ0.1mol��
��22.4L/mol=0.56L����ʱ������ij�缫����2.8gij������ת�Ƶ���Ϊ0.1mol��
A.���MgSO4��Һ�����ݵ����������ӷŵ�˳���������ȷŵ磬���������������ʣ���A���������⣻
B.���CuSO4��Һ�����ݵ����������ӷŵ�˳��ͭ�����ȷŵ磬1molͭ���ӵõ�2mol���ӱ�Ϊ1molͭ����64g��ת�Ƶ���Ϊ0.1mol������ͭ����3.2g������Һ��ͭ�������ʵ���С��![]() =0.044mol������ܵõ�2.8g��������B�������⣻
=0.044mol������ܵõ�2.8g��������B�������⣻
C.���NaCl��Һ�����ݵ����������ӷŵ�˳���������ȷŵ磬���������������ʣ���C���������⣻
D.���AgNO3��Һ�����ݵ����������ӷŵ�˳���������ȷŵ磬1mol�����ӵõ�1mol���ӱ�Ϊ1mol������108g��ת�Ƶ���Ϊ0.1mol�������Ƶ���10.8g������Һ�����������ʵ�������![]() =0.026mol������ܵõ�2.8g��������D�������⣻
=0.026mol������ܵõ�2.8g��������D�������⣻
����0.56��BD��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ����ͬ�¶��£������Ϊ0.25 L�����������ܱ������з������淴Ӧ��N2(g)��3H2(g) ![]() 2NH3(g)����H����92.6 kJ��mol��1��ʵ������ʼ��ƽ��ʱ���й��������±���ʾ��
2NH3(g)����H����92.6 kJ��mol��1��ʵ������ʼ��ƽ��ʱ���й��������±���ʾ��
������� | ��ʼʱ�����ʵ����ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | ||
N2 | H2 | NH3 | ||
�� | 1 | 3 | 0 | �ų�������23.15 kJ |
�� | 0.9 | 2.7 | 0.2 | �ų�������Q |
���������������
A.�����١����з�Ӧ��ƽ�ⳣ�����
B.ƽ��ʱ������������NH3�����������Ϊ![]()
C.�������д�ƽ��ʱ�ų�������Q��23.15 kJ
D.�������ٵ����Ϊ0.5 L����ƽ��ʱ�ų�������С��23.15 kJ
����Ŀ����֪��2CH3OH(g)![]() CH3OCH3(g)��H2O(g)����H����25 kJ��mol��1��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���������ܱ������м���CH3OH��ijʱ�̲�ø���ֵ����ʵ���Ũ�����±�������˵���в���ȷ����(����)
CH3OCH3(g)��H2O(g)����H����25 kJ��mol��1��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���������ܱ������м���CH3OH��ijʱ�̲�ø���ֵ����ʵ���Ũ�����±�������˵���в���ȷ����(����)
���� | CH3OH | CH3OCH3 | H2O |
c/mol��L��1 | 0.08 | 1.6 | 1.6 |
A. ��ʱ�̷�Ӧ�ﵽƽ��״̬
B. ������ѹǿ����ʱ��˵����Ӧ��ƽ��״̬
C. ƽ��ʱ���ټ�������ʼ������CH3OH������ƽ���CH3OHת���ʲ���
D. ƽ��ʱ����Ӧ����������������40 kJ