��Ŀ����
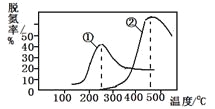
����Ŀ����ͼA��B��C��D��E��F�ȼ��ֳ����л���֮���ת����ϵͼ������A����۵���Ҫ�ɷ֣�C��E��Ӧ������F��F������ζ��
![]()
���л����У����Ǿ�����CHO�ṹ�����ʣ������������ʣ�
��������������ͭ����Һ��Ӧ������ש��ɫ������
���ڴ��������£���CHO����������Ϊ��COOH����![]() ��
��
����������Ϣ�������ʵ�ת����ϵ������и��⡣
��1��B�Ļ�ѧʽΪ_________��C�Ľṹ��ʽΪ__________��
��2��������������������ͭ����Һ��Ӧ����ש��ɫ������������_________�������ƣ���
��3��C��D�Ļ�ѧ����ʽΪ______________��
��4��C + E�� F�Ļ�ѧ����ʽΪ ______________��
���𰸡�C6H12O6 CH3CH2OH �����ǡ���ȩ ![]()
![]()
��������
AΪ��۵���Ҫ�ɷ֣���AΪ���ۣ�����ˮ�����������ǣ���BΪ�����ǣ�C6H12O6���������Ƿ��������Ҵ�����CΪ�Ҵ���C2H5OH�� ���Ҵ���ͭ���������������±�����������ȩ����DΪ��ȩ��CH3CHO������ȩ���ű������������ᣬ��EΪ���ᣨCH3COOH�����������Ҵ�����������Ӧ����F��FΪ�������� ��CH3COOCH2CH3����
��1��BΪ�����ǣ������ǵķ���ʽΪ C6H12O6��CΪ�Ҵ����Ҵ��Ľṹ��ʽΪCH3CH2OH���ʴ�Ϊ��C6H12O6��CH3CH2OH��
��2�����л����У����Ǿ�����CHO�ṹ�����ʣ�������������ͭ����Һ��Ӧ������ש��ɫ�����������ǡ���ȩ����ȩ�������ܱ�����Cu(OH)2����������ש��ɫ�������ʴ�Ϊ�������ǡ���ȩ��
��3��CΪ�Ҵ�����ͭ���������������±�����������ȩ���仯ѧ��Ӧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��4��CΪ�Ҵ���EΪ���ᣬ�������Ҵ�����������Ӧ������������ ��CH3COOCH2CH3����ˮ����ѧ��Ӧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�����Ŀ��ʵ����Ϊ̽������Ũ����(����)�ķ�Ӧ������֤SO2�����ʣ������ͼ��ʾװ�ý���ʵ�飬����˵������ȷ����( )
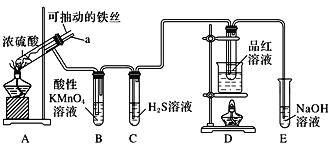
A��װ��B������KMnO4��Һ����ɫ�������˶�������Ļ�ԭ�� |
B��ʵ����������װ��A����Һ�еμ�KSCN��Һ�Լ������ɵ�Fe3+ |
C��װ��D��Ʒ����Һ��ɫ������֤SO2��Ư���� |
D��ʵ��ʱ������a����Ũ�����У��ɷ�ֹװ��B�е���Һ���� |