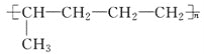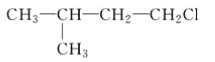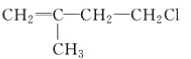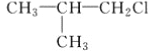��Ŀ����
����Ŀ��Ϊ�����Դ��ȱ���⣬��ҵ������Ӧ�������û�ѧ�ܡ�
��1��25�棬1.01��105Paʱ��ʵ���ã�4g������O2����ȫȼ������Һ̬ˮ���ų�572kJ�����������ʾH2��ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________________��
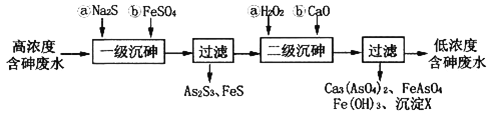
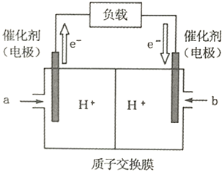
��2����ͼ��ij�ʼDZ�����ʹ�õļ״�ȼ�ϵ�صĽṹʾ��ͼ���ŵ�ʱ�״�Ӧ��_________��ͨ�루�a����b����������ڲ�H+��_________������ҡ����ƶ���д����ظ����ĵ缫��Ӧʽ_________________________________��
��3���ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡�
��ѧ�� | H-H | N-H | N��N |
����/ kJ��mol-1 | 436 | a | 945 |
��֪��N2(g)+3H2(g)=2NH3(g) ��H=-93 kJ��mol-1���Ը��ݱ������м������ݼ���a����ֵ__________��
��4����֪��C��s��ʯī��+O2(g)=CO2(g) ��H1=-393.5 kJ��mol-1 ��
2H2(g)+O2(g)=2H2O(1) ��H2=-571.6 kJ��mol-1 ��
2C2H2(g)+5O2(g)=4CO2(g)+2H2O(1) ��H3=-2599 kJ��mol-1 ��
���ݸ�˹���ɣ����㷴Ӧ2C��s��ʯī��+H2(g)=C2H2(g)�ġ�H=_______________��
���𰸡� H2(g)+![]() O2(g)=H2O(1) ��H=-286kJ/mol a �� CH3OH+H2O-6e-=CO2+6H+ 391 +226.7kJ��mol-1
O2(g)=H2O(1) ��H=-286kJ/mol a �� CH3OH+H2O-6e-=CO2+6H+ 391 +226.7kJ��mol-1
��������(1)ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�������������n=![]() ����1mol������ȫȼ�շų�������������Ȼ�ѧ����ʽ����д����д���Ȼ�ѧ����ʽ��(2)���ݵ��������֪aΪ��������������������Ӧ��bΪ����������ԭ��Ӧ����Һ������������������(3)���ݷ�Ӧ�ȵ��ڷ�Ӧ����ܼ���-��������ܼ���������(4)���ø�˹���ɷ������
����1mol������ȫȼ�շų�������������Ȼ�ѧ����ʽ����д����д���Ȼ�ѧ����ʽ��(2)���ݵ��������֪aΪ��������������������Ӧ��bΪ����������ԭ��Ӧ����Һ������������������(3)���ݷ�Ӧ�ȵ��ڷ�Ӧ����ܼ���-��������ܼ���������(4)���ø�˹���ɷ������
(1)4g������2mol��O2����ȫȼ������Һ̬ˮ���ų�572kJ����������1mol������������ȼ������Һ̬ˮ���ų�286kJ�����������ʾH2��ȼ���ȵ��Ȼ�ѧ����ʽΪ��H2(g)+![]() O2(g)�TH2O(l)��H=-286kJ/mol���ʴ�Ϊ��H2(g)+
O2(g)�TH2O(l)��H=-286kJ/mol���ʴ�Ϊ��H2(g)+ ![]() O2(g)�TH2O(l)��H=-286kJ/mol��
O2(g)�TH2O(l)��H=-286kJ/mol��
(2)ԭ����е��ӴӸ��������·�������������ݵ��������֪a�缫Ϊ�������״��ڸ����Ϸ���������Ӧ���缫��ӦʽΪCH3OH+H2O-6e-�TCO2+6H+��bΪ�����������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ3O2+12e-+12H+�T6H2O�����������������Ҳ࣬�ʴ�Ϊ��a���ң�CH3OH+H2O-6e-�TCO2+6H+��
(3)N2(g)+3H2(g)2NH3(g)��H=945kJmol-1+436kJmol-1��3-akJmol-1��6=-93kJmol-1��a=391 kJmol-1���ʴ�Ϊ��391��
(4)��C(s��ʯī)+O2(g)�TCO2(g)��H=-393.5kJmol-1����2H2(g)+O2(g)�T2H2O(l)��H=-571.6kJmol-1����2C2H2(g)+5O2(g)�T4CO2(g)+2H2O(l)��H=-2599kJmol-1�����ø�˹���ɽ�����2+����![]() -����
-����![]() �ɵã�2C(s��ʯī)+H2(g)=C2H2(g)��H=(-393.5kJ/mol)��2+
�ɵã�2C(s��ʯī)+H2(g)=C2H2(g)��H=(-393.5kJ/mol)��2+![]() ��(-571.6kJ/mol)-
��(-571.6kJ/mol)- ![]() ��(-2599 kJ/mol)=+226.7kJmol-1���ʴ�Ϊ��+226.7kJmol-1��
��(-2599 kJ/mol)=+226.7kJmol-1���ʴ�Ϊ��+226.7kJmol-1��

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�����Ŀ����һ�����ļ��Ȼ��[(CH3)4NCl]ˮ��ҺΪԭ�ϣ�ͨ����ⷨ�����Ʊ��ļ��������[(CH3)4NOH]��װ����ͼ��ʾ��

��1���ռ���(CH3)4NOH��������________(��a��b��c��d)��
��2��д������ܷ�Ӧ_____________��
��������������һ��ͨ��������Ҵ������ϳɣ�CH3COOH(l)+C2H5OH(l) ![]() CH3COOC2H5(l)+H2O(l) ��H=��2.7kJ��mol��1
CH3COOC2H5(l)+H2O(l) ��H=��2.7kJ��mol��1
��֪�����ʺ���غ�л����ij�ѹ�е����±���
������ | �е�/�� | ��л������������� | �е�/�� |
�Ҵ� | 78.3 | ��������(0.92)+ˮ(0.08) | 70.4 |
���� | 117.9 | ��������(0.69)+�Ҵ�(0.31) | 71.8 |
�������� | 77.1 | ��������(0.83)+�Ҵ�(0.08) +ˮ(0.09) | 70.2 |
����ɣ�
��1�����ڸ÷�Ӧ������˵������������_____________��
A����Ӧ��ϵ�������д�����
B����Ϊ��ѧ����ʽǰ�����ʵĻ�ѧ������֮����ȣ����Է�Ӧ�Ħ�S������
C����Ϊ��Ӧ�ġ�H �ӽ����㣬�����¶ȱ仯��ƽ��ת���ʵ�Ӱ���
D����Ϊ��Ӧǰ����Һ̬���ʣ�����ѹǿ�仯�Ի�ѧƽ���Ӱ��ɺ��Բ���
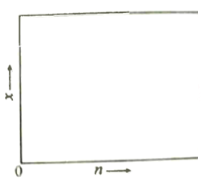
��2��һ���¶��¸÷�Ӧ��ƽ�ⳣ��K=4.0��������ѧ����ʽ��������Ҵ��Ļ�ѧ����������Ͷ�ϣ�������������ƽ�����y =_________����������Ҵ������ʵ���֮��Ϊn : 1����Ӧƽ����ϵ���������������ʵ�������Ϊx������ͼ�л���x��n�仯��ʾ��ͼ(����ʱ���Ƹ���Ӧ)________��
��3����ҵ�϶������������ķ��������ϳ��������ᡢ�Ҵ���������Һ������110�����ҷ���������Ӧ��������ֱ�������¶ȴﵽ70��71�棬��ʼ���������ϡ��������������������___________________��
��4�����꣬��ѧ���о����Ҵ����ϳ������������·�����2C2H5OH(g) ![]() CH3COOC2H5(g)+2H2(g)
CH3COOC2H5(g)+2H2(g)
�ڳ�ѹ�·�Ӧ�������ռ�����ó�����Һ���ռ�������Ҫ���������������ͼ��ʾ�����ڸ÷����������Ʋ��������_____��
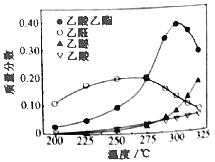
A����Ӧ�¶Ȳ��˳���300��
B��������ϵѹǿ������������Ҵ�ƽ��ת����
C���ڴ��������£���ȩ�Ƿ�Ӧ�����е��м����
D����ߴ����Ļ��Ժ�ѡ���ԣ��������ѡ���ϩ�ȸ������ǹ��յĹؼ�
����Ŀ����Ҫ����д
(1)�����ĵ���ʽ��_________����Ȳ�Ľṹ��ʽ��___________��
(2)��ҵ��������ϩ��ˮ������ȡ�Ҵ�����ѧ��Ӧ����ʽ��________��Ӧ���ͣ�____________
(3)����Ũ������50~60���·���������Ӧ��________��Ӧ���ͣ�_____________��
(4)��֪�������£�
��ѧ�� | H��H | N��N | N��H | N��N |
����kJ/mol | 432 | 247 | 389 | 942 |
д��N2��H2�ϳ�NH3���Ȼ�ѧ����ʽ��_____________��