��Ŀ����
T��ʱ���мס��������ܱ������������������Ϊ1 L�������������Ϊ2 L���ֱ���ס����������м���6 mol A��3 mol B��������Ӧ���£�3A(g)��bB(g) 3C(g)��2D(g)����H<0; 4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol/L��B��Ũ��Ϊ1.8 mol/L; t minʱ�������ڵķ�Ӧ��ƽ�⣬B��Ũ��Ϊ0.8 mol/L.���������Ϣ�ش��������⣺
(1)�������з�Ӧ��ƽ������v(B)��________����ѧ����ʽ�м�����b��________.
(2)�������з�Ӧ�ﵽƽ��ʱ����ʱ��t________4min(����ڡ�����С�ڡ����ڡ�)��ԭ����______________________________��
(3)T��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8mol/L����ʼʱ����������м���C��D�����ʵ����ֱ�Ϊ3 mol��2 mol���������A��B�����ʵ����ֱ���________��________.
(4)��Ҫʹ�ס���������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��________��
A�������¶Ȳ��䣬����������������2 L
B����������������䣬ʹ�����������¶�
C����������ѹǿ���¶ȶ����䣬����м���һ������A����
D����������ѹǿ���¶ȶ����䣬����м���һ������B����
(5)д��ƽ�ⳣ������ʽK��________����������T��ʱ�Ļ�ѧƽ�ⳣ��K��________.
(1)0.3 mol/(L��min)��1
(2)���ڡ���������������ڼ������������Ũ�ȼ�С ����Ӧ���ʼ�������ƽ������ʱ�䳤
(3)3 mol��2 mol��(4)AC
(5) ��10.8 mol/L
����:����3A(g)��bB(g) 3C(g)��2D(g)����H<0;
6 mol 3 mol 0 0
3.6mol 1.2mol 3.6mol 2.4mol
2.4mol 1.8mol 1.2mol 0.8mol
b=1 v(B)��0.3mol/(L��min)
����3A(g)��B(g) 3C(g)��2D(g)����H<0;
6 mol 3 mol 0 0
4.2mol 1.4mol 4.2mol 0.7mol
1.8mol 1.6mol 4.2mol 0.7mol
ABŨ�Ȳ��ϼ�С��t����4 min
�ɵ�Чƽ��ó�����A��B�����ʵ����ֱ���3 mol��2 mol��

 3C��g����2D��g����
3C��g����2D��g����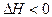 ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺ 3C(g)��2D(g)����H<0; 4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol/L��B��Ũ��Ϊ1.8 mol/L; t minʱ�������ڵķ�Ӧ��ƽ�⣬B��Ũ��Ϊ0.8 mol/L.���������Ϣ�ش��������⣺
3C(g)��2D(g)����H<0; 4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol/L��B��Ũ��Ϊ1.8 mol/L; t minʱ�������ڵķ�Ӧ��ƽ�⣬B��Ũ��Ϊ0.8 mol/L.���������Ϣ�ش��������⣺ 3C��g����2D��g����
3C��g����2D��g���� ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺