��Ŀ����
��6�֣�T��ʱ���мס��������ܱ������������������Ϊ1L�������������Ϊ2L���ֱ���ס����������м���6mol A��3mol
B��������Ӧ���£�3A��g����b B��g�� 3C��g����2D��g����
3C��g����2D��g���� ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��1���������з�Ӧ��ƽ������v��B����_________����ѧ����ʽ�м�����b��________��
��2���������з�Ӧ�ﵽƽ������ʱ��t_________4min������ڡ�����С�ڡ����ڡ�����ԭ����___________________________________________________________��
��3��T��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8mol/L����ʼʱ����������м���C��D�����ʵ����ֱ�Ϊ3mol��2mol���������A��B�����ʵ����ֱ���___________��___________��
��ÿ��1�֣���6�֣�
��1��0.3mol/(L��min)��1
��2�����ڣ���������������ڼ��������������Ӧ��Ũ�ȼ�С����Ӧ���ʼ�����
��ƽ������ʱ�䳤
��3��3mol��2mol
��������

 ��У����ϵ�д�
��У����ϵ�д� 3C��g����2D��g����
3C��g����2D��g����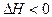 ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺ 3C(g)��2D(g)����H<0; 4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol/L��B��Ũ��Ϊ1.8 mol/L; t minʱ�������ڵķ�Ӧ��ƽ�⣬B��Ũ��Ϊ0.8 mol/L.���������Ϣ�ش��������⣺
3C(g)��2D(g)����H<0; 4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol/L��B��Ũ��Ϊ1.8 mol/L; t minʱ�������ڵķ�Ӧ��ƽ�⣬B��Ũ��Ϊ0.8 mol/L.���������Ϣ�ش��������⣺