��Ŀ����
1���ܣ�Co�����仯�����ڹ�ҵ���й㷺Ӧ�ã�Ϊ��ij��ҵ�����л����ܣ�ijѧ�����������ͼ�������к���Al��Li��Co2O3��Fe2O3�����ʣ���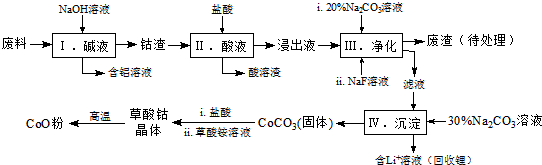
��֪���������ܽ��ԣ�LiF������ˮ��Li2CO3����ˮ��
�ڲ��ֽ��������γ��������������pH������
| Fe3+ | Co2+ | Co3+ | Al3+ | |
| pH����ʼ������ | 1.9 | 7.15 | -0.23 | 3.4 |
| pH����ȫ������ | 3.2 | 9.15 | 1.09 | 4.7 |
��1��д���������Co2O3�����ᷴӦ����Cl2�����ӷ���ʽ��Co2O3+6H++2Cl-=2Co2++Cl2��+3H2O��
��2��������� Na2CO3��Һ�������ǵ�����Һ��pH��Ӧʹ��Һ��pH�������������е���Ҫ�ɷֳ���LiF�⣬����Fe��OH��3��
��3��NaF ����Һ�е�Li+�γ�LiF�������˷�Ӧ�Բ��������������ǽ�����Һ��Li+Ũ�ȣ����ⲽ����в���Li2CO3������
��4���ڿ����м���5.49g�����ܾ��壨CoC2O4•2H2O����Ʒ�����ȹ����в�ͬ�¶ȷ�Χ�ڷֱ�õ�һ�ֹ������ʣ��������������֪��M��CoC2O4•2H2O��=183g/mol
| �¶ȷ�Χ/�� | ��������/g |
| 150��210 | 4.41 |
| 290��320 | 2.41 |
| 890��920 | 2.25 |
���� ���ϼ�������������Һ������õ����������������ܽ���ɹ˪������ԭ��Ӧ������������������Ϊ���������˵õ����ܵ���Һ����̼������Һ��NaF��Һ��������ȥ����Ӻ������ӣ�����Һ�м���̼������Һ����������Ϊ̼���ܳ�������������Ͳ������Һ�õ������ܾ��壬���·ֽ�õ������ܣ�
��1������������������Һ���Ȼ���Ϊ���������ԭ���غ�͵����غ������ƽ��д���ӷ���ʽ��
��2�����ݱ������ݷ����жϼ���̼���Ƶ�����ҺPH�����������Ӻ�����ӣ������ܳ��������ӣ�
��3��NaF����Һ�е�Li+�γ�LiF��������ֹ����ӽ��̼��������γ�̼��﮳�����
��4�����㾧�����ʵ���n=$\frac{5.49g}{183g/mol}$=0.03mol��ʧȥ�ᾧˮӦΪ0.06mol�����������仯=0.06mol��18g/mol=1.08g��ͼ�����ݿ�֪��150��210���������仯=5.49g=4.41g=1.08g��˵��210��Cʧȥ�ᾧˮ�õ�CoC2O4��210��290���������CoC2O4�����ķ�Ӧ��210��290������в���������ֻ��CO2 ������Ԫ���غ�õ�����CO2���ʵ���Ϊ0.06mol������=0.06mol��44g/mol=2.64g�������������Ƽ�С=4.41g-2.41g=2g��˵�����ǷֽⷴӦ���μӷ��õĻ�����������Ӧ����������=2.64g-2g=0.64g��O2���ʵ���=$\frac{0.64g}{32g/mol}$=0.02mol������ԭ���غ���ƽ��д��Ӧ�Ļ�ѧ����ʽ���¶ȸ���890��ʱ�����������ֽⷴӦ���õ����������Ϊ2.25g�����к�����Ԫ�ص�����Ϊ0.03��59g=1.77g�����Թ����к�����Ԫ�ص�����Ϊ0.48g�������ʵ�����0.03mol���ݴ˴��⣻
��� �⣺��1������������������Һ���Ȼ���Ϊ���������ԭ���غ�͵����غ������ƽ��д���ӷ���ʽ����Ԫ�ػ��ϼ۽���Ϊ+2�ۣ���Ԫ�ػ��ϼ�-1�۱仯Ϊ0�ۣ���Ӧ�����ӷ���ʽ��Co2O3+6H++2Cl-=2Co2++Cl2��+3H2O
�ʴ�Ϊ��Co2O3+6H++2Cl-=2 Co2++Cl2��+3H2O��
��2�����ݱ������ݷ����жϼ���̼���Ƶ�����ҺPH�����������Ӻ�����ӣ������ܳ��������ӣ��������Na2CO3��Һ�������ǵ�����Һ��pH��Ӧʹ��Һ��pH������7.15�������е���Ҫ�ɷֳ���LiF���Fe��OH��3��
�ʴ�Ϊ��7.15��Fe��OH��3��
��3��NaF����Һ�е�Li+�γ�LiF�������˷�Ӧ�Բ��������������ǣ�������Һ��Li+Ũ�ȣ����ⲽ����в���Li2CO3������
�ʴ�Ϊ��������Һ��Li+Ũ�ȣ����ⲽ����в���Li2CO3������
��4�����㾧�����ʵ���n=$\frac{5.49g}{183g/mol}$=0.03mol��ʧȥ�ᾧˮӦΪ0.06mol�����������仯=0.06mol��18g/mol=1.08g��ͼ�����ݿ�֪��150��210���������仯=5.49g=4.41g=1.08g��˵��210��Cʧȥ�ᾧˮ�õ�CoC2O4��210��290���������CoC2O4�����ķ�Ӧ��210��290������в���������ֻ��CO2 ������Ԫ���غ�õ�����CO2���ʵ���Ϊ0.06mol������=0.06mol��44g/mol=2.64g�������������Ƽ�С=4.41g-2.41g=2g��˵�����ǷֽⷴӦ���μӷ��õĻ�����������Ӧ����������=2.64g-2g=0.64g��O2���ʵ���=$\frac{0.64g}{32g/mol}$=0.02mol��n��CoC2O4����n��O2����n��CO2��=0.03��0.02��0.06=3��2��6������ԭ���غ���ƽ��д��Ӧ�Ļ�ѧ����ʽΪ3CoC2O4+2O2 $\frac{\underline{\;210��-290��\;}}{\;}$Co3O4+6CO2���¶ȸ���890��ʱ�����������ֽⷴӦ���õ����������Ϊ2.25g������ǰ��ļ����֪��������ֻ������Ԫ�غ���Ԫ�أ����к�����Ԫ�ص�����Ϊ0.03��59g=1.77g�����Թ����к�����Ԫ�ص�����Ϊ0.48g�������ʵ�����0.03mol�����Թ�������ԭ������ԭ�ӵ����ʵ���֮��Ϊ0.03��0.03=1��1�����Թ���Ļ�ѧʽΪCoO��
�ʴ�Ϊ��3CoC2O4+2O2 $\frac{\underline{\;210��-290��\;}}{\;}$Co3O4+6CO2��CoO��
���� ���⿼�������̷����жϣ��������ʵ�����Ӧ�ã���Ҫ�ǹ������ȹ����������仯�����㼰��ѧ����ʽ��д�����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| A�� | ������м��ٵ�3 g����һ���ǻ���� | |
| B�� | ��������������ٵ�����һ����Cu | |
| C�� | ���������������Եó�m��Fe2O3����m��Cu��=1��1 | |
| D�� | ���ݲ��������жϻ����X�ijɷ�ΪAl2O3��Fe2O3��Cu��SiO2 |
| A�� | ����״̬�µ�NaHSO4���룺NaHSO4�TNa++H++SO${\;}_{4}^{2-}$ | |
| B�� | Fe��OH��3�ĵ��룺Fe��OH��3?Fe3++3OH- | |
| C�� | H2CO3�ĵ��룺H2CO3?2H++CO${\;}_{3}^{2-}$ | |
| D�� | ˮ��Һ�е�NaHSO4���룺NaHSO4�TNa++HSO${\;}_{4}^{-}$ |
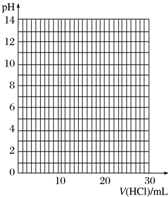 ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ����ش��������⣺
ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ����ش��������⣺��1����ͬѧ���ó������ⶨ��Ʒ��NaOH�ĺ�������ͬѧѡ�õ�ҩƷ����Ʒ�⣬��Ӧ���Ȼ�����Һ��ʵ����Ӧ�ⶨ����������Ʒ�����ͳ�����������
��2����ͬѧ���õζ����ⶨ��Ʒ��NaOH�ĺ�����
���÷�����ƽ��ȡ����Ʒ5.000g��ȫ������ˮ���Ƴ�1 000.0mL��Һ���ü�ʽ�ζ�����ȡ20.00mL������Һ������ƿ�У��μӼ���ָʾ�������⣮�ζ�����ʹ��ǰ��ϴ���⣬��Ӧ��©����ϴ��
����Ũ��Ϊ0.100 0mol•L-1���������Һ���еζ�����ʼ�ζ�ǰ��һ�������ǵ���Һ���ڡ�0���̶Ȼ�0���̶����£�
�۵ζ���������pH�Ʋⶨ��ƿ����Һ��pH���ٽ��ζ��յ�ʱ�ⶨpHӦÿ��һ�β�һ�Σ�
�ܵζ������У���ƿ����Һ��pH�仯���£�
| V��HCl��/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
| ָʾ�� | ��ɫ��Χ��pH�� | ��ɫ | |
| �� | �� | ||
| ���� | 3.1��4.4 | �� | �� |
| ʯ�� | 5.0��8.0 | �� | �� |
| ��̪ | 8.2��10.0 | �� | �� |
����Ʒ�У�NaOH�������ٷֺ���Ϊ96%��
��3�����µζ������ܵ������յζ����ƫ�͵���ADF
A����ʽ�ζ���ȡҺǰ���촦�����ݣ�ȡҺ��������ʧ
B���ζ����������Ӷ�ȡ��ʽ�ζ��ܵ�����
C����ƿ��ʢװ����Һ֮ǰ����������ˮ
D���ζ�ʱ����ƿ��ҡ����������Һ��ɽ�����
E����ʽ�ζ���ʹ��ǰδ��ϴ
F����ʽ�ζ���ʹ��ǰδ��ϴ��
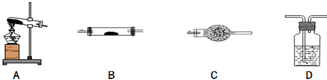 ������������Ļ�ѧʽΪFeC2O4•2H2O������һ�ֵ���ɫ�ᾧ��ĩ�������̼��ԣ�����ʱ�ɷ������·ֽⷴӦ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeO+CO2��+CO��+2H2O
������������Ļ�ѧʽΪFeC2O4•2H2O������һ�ֵ���ɫ�ᾧ��ĩ�������̼��ԣ�����ʱ�ɷ������·ֽⷴӦ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeO+CO2��+CO��+2H2O��1��������ͼ���ṩ�������������ظ�ѡ�ã���ÿ����������ѡ��һ�Σ���ѡ���Ҫ���Լ�[��ѡ����Լ���NaOH��Һ������ʯ��ˮ������̼������Һ��CuO����ˮ����ͭ�����Ը��������Һ]�����һ��ʵ�飬����FeC2O4•2H2O����ʱ�ֽ��������̬�������ˮ�����������ּ���װ�úͼг�������ͼ����ȥ�����ڴ������д�����Բ���������Ҳ���Բ��䣩��
| �������� | �������������� | ���� |
| A | ������������ | ���Ȳ�����������õ�������� |
| D | NaOH��Һ | |
��3����Ӧ��������Aװ���Թ����к�ɫ�����ĩ����������������ʱ����ȼ����������������Ŀ���ԭ����FeC2O4•2H2O�ֽ������CO������FeO��ԭΪ��ĩ״�ĵ����������۱������е�����������ȼ�գ�
| A�� | ��Ӿ�����֤���������� | |
| B�� | ������һ�ִ����� | |
| C�� | �������ڽ�����ϵ | |
| D�� | �������Ȼ�����Һ��������������Һ���Ʊ������������� |
| A�� | 0.5molп�����������ᷴӦ����11.2L H2 | |
| B�� | ��״���£�11.2L H2O����������Ϊ0.5NA | |
| C�� | 0.5mol•L-1��MgCl2��Һ�к���Cl-����ΪNA | |
| D�� | 25�桢101Paʱ��16g O2��O3��������к��е�ԭ����ΪNA |
| A�� | 31 g������P-P��������Ϊ6 NA | |
| B�� | 1.6 g NH2?���Ӻ��е�������ΪNA | |
| C�� | 1 L1 mol•L-1FeCl3��Һ�к���Fe3+����ĿΪNA | |
| D�� | ��״����22.4 L NO�� 22.4 L O2�Ļ����������������Ϊ1.5NA |