��Ŀ����
 ��ͼΪһ���绯ѧ���̵�ʾ��ͼ����ش��������⣺
��ͼΪһ���绯ѧ���̵�ʾ��ͼ����ش��������⣺��1��ͨ��CH30Hһ���ĵ�ⷴӦʽΪ
��2���ҳ���ͭ�ľ����أ���A�缫�IJ�����
��3�������к���0.01mol CuSO4��0.01mol NaCl�Ļ����Һ100mL�������·��ת����0.02mol e-�������������������ڱ�״���µ������
��4�������е��з�̪��ʵ�鿪ʼ��۲쵽��������
���㣺ԭ��غ͵��صĹ���ԭ��
ר�⣺�����ӵ������Ͱ����ӵ�����
��������1���״���CԪ�صĻ��ϼ����ߣ���ͨ��CH3OH�ĵ缫Ϊ������ʧȥ���ӷ���������Ӧ��
��2������A��������������AΪ��������ͭ������ͭΪ�����������Ϊ������ͭ�Σ�
��3�������·��ת����0.02mol e-�������ֱ�����������������
��4������Ȼ�����Һ�������������������������أ���Һ�ʼ��ԣ�
��2������A��������������AΪ��������ͭ������ͭΪ�����������Ϊ������ͭ�Σ�
��3�������·��ת����0.02mol e-�������ֱ�����������������
��4������Ȼ�����Һ�������������������������أ���Һ�ʼ��ԣ�
���
�⣺��1���״���CԪ�صĻ��ϼ����ߣ���ͨ��CH3OH�ĵ缫Ϊ������ʧȥ���ӷ���������Ӧ���缫��ӦΪCH3OH-6e-+8OH-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-�TCO32-+6H2O��
��2������A��������������AΪ��������ͭ������ͭΪ��������AΪ��ͭ�������Ϊ������ͭ�Σ���ѡ����ͭ��Һ��B�缫Ϊ����������Cu2++2e-=Cu��
�ʴ�Ϊ����ͭ��Cu2++2e-�TCu��
��3�������·��ת����0.02mol e-�������ֱ�Ϊ��2Cl--2e-=Cl2����4OH--4e-=2H2O+O2�����ֱ�����0.005molCl2��0.0025molO2�������Ϊ0.0075mol��22.4L/mol=0.168L=168mL��
�ʴ�Ϊ��168 mL��
��4������Ȼ�����Һ�������������������������أ���Һ�ʼ��ԣ��ɹ۲쵽���缫��������ð�����ҵ缫������Һ��죬����ܷ�ӦʽΪ2KCl+2H2O
2KOH+H2��+Cl2����
�ʴ�Ϊ�����缫��������ð�����ҵ缫������Һ��죻2KCl+2H2O
2KOH+H2��+Cl2����
�ʴ�Ϊ��CH3OH-6e-+8OH-�TCO32-+6H2O��
��2������A��������������AΪ��������ͭ������ͭΪ��������AΪ��ͭ�������Ϊ������ͭ�Σ���ѡ����ͭ��Һ��B�缫Ϊ����������Cu2++2e-=Cu��
�ʴ�Ϊ����ͭ��Cu2++2e-�TCu��
��3�������·��ת����0.02mol e-�������ֱ�Ϊ��2Cl--2e-=Cl2����4OH--4e-=2H2O+O2�����ֱ�����0.005molCl2��0.0025molO2�������Ϊ0.0075mol��22.4L/mol=0.168L=168mL��
�ʴ�Ϊ��168 mL��
��4������Ȼ�����Һ�������������������������أ���Һ�ʼ��ԣ��ɹ۲쵽���缫��������ð�����ҵ缫������Һ��죬����ܷ�ӦʽΪ2KCl+2H2O
| ||
�ʴ�Ϊ�����缫��������ð�����ҵ缫������Һ��죻2KCl+2H2O
| ||
���������⿼��ԭ��غ͵��ԭ������ȷ���ӵķŵ�˳�����ĵ缫��Ӧ�ǽ��Ĺؼ�����ע�����õ����غ���������̣���Ŀ�Ѷ��еȣ���4��Ϊ�����ѵ㣮

��ϰ��ϵ�д�
�����Ŀ
����ͼװ���У�aΪFe��bΪ̼�������ڴ�װ�õĸ���������ȷ���ǣ�������


| A��a�缫��������ų���b�缫�������ݣ���ҺpH��� |
| B��a��������b�Ǹ��� |
| C���������е������������·�У����Ӵ�a����b�� |
| D��a���Ϸ����˻�ԭ��Ӧ |
���г��ӷ�����ȷ���ǣ�������Ϊ���Ӽ�����������
| A��NaOH��Һ�л���Ba��OH��2��K2SO4�� |
| B��NaCl��Һ�л���Na2CO3�����ᣩ |
| C��CO�л���CO2�����ȵ�̿�� |
| D��CO2�л���HCl���壨NaOH��Һ�� |
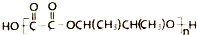 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼ��ͼ��
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼ��ͼ��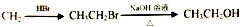
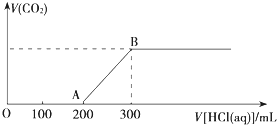 ��100mL 3mol?L-1��NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ����������Һ��pH��7��
��100mL 3mol?L-1��NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ����������Һ��pH��7�� ����������ԭ��Ӧ��Zn��s��+2Ag+��aq���TZn2+��aq��+2Ag��s���������һ����ԭ��أ�
����������ԭ��Ӧ��Zn��s��+2Ag+��aq���TZn2+��aq��+2Ag��s���������һ����ԭ��أ�