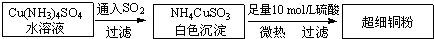��Ŀ����
S��N��Cl������Ҫ�ķǽ���Ԫ�أ�����˵����ȷ��
A��98%��Ũ�����õ������ˮϡ�ͺ��������������Ϊ49%
B��SO32����ClO����NO3��������������Һ������������ԭ��Ӧ�����ܴ�������
C��Ũ���ᡢ�������ǿ�����ԣ�Ũ�����Ũ�����ֽ���в��ȶ���
D������£�22.4L��������������������Һ��Ӧ��ת�Ƶĵ�����Ϊ2NA��(NAΪ�����ӵ�����)
���𰸡�
B
��������
���������A��98%��Ũ�����õ������ˮϡ�ͺ�����ˮ���ܶȺ�������ܶȲ�ͬ�������������������Ϊ����49%��C��Ũ����ֽ⣬���ӷ�������D��ת�Ƶĵ�����ΪNA��������
���㣺������Һ��ϡ�ͣ��������ʵ����ʺ�NA�����֪ʶ��

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
S��N��Cl������Ҫ�ķǽ���Ԫ�أ�����˵����ȷ�ǣ�������
| A��98%��Ũ�����õ������ˮϡ�ͺ��������������Ϊ49% | B��SO32-��ClO-��NO3-������������Һ������������ԭ��Ӧ�����ܴ������� | C��Ũ���ᡢ�������ǿ�����ԣ�Ũ�����Ũ�����ֽ���в��ȶ��� | D������£�22.4L��������������������Һ��Ӧ��ת�Ƶĵ�����Ϊ2NA����NAΪ�����ӵ������� |
 ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�