��Ŀ����
12��X��Y��Z��W���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶W��Z��X��Y������X��Y����ͬһ���ڣ�X��Z����ͬһ���壮Zԭ�Ӻ�������������X��Y��ԭ�Ӻ���������֮�ͣ�Zԭ��������ϵĵ�������Wԭ��������������4�����Ը������������ش���1��д��������Ԫ�����ƣ�X̼��Y����Z�裬W�ƣ�
��2��ZԪ�������ڱ��е�λ�õ�3���ڵڢ�A��
��3��������Ԫ���еķǽ����⻯����ȶ����ɴ�С��˳����H2O��CH4��SiH4��NaH�����û�ѧʽ��ʾ��
��4��X��Y�γɵ���ԭ�ӷ��ӵĵ���ʽ��
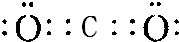 ��Y��W�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ��
��Y��W�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ��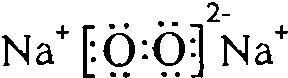 ��
����5��WԪ������������Ӧˮ����Ļ�ѧʽ��NaOH���볣���������������ﷴӦ�Ļ�ѧ����ʽΪNaOH+Al��OH��3=NaAlO2+2H2O��
���� X��Y��Z��W���Ƕ���������Ԫ�أ�ԭ�Ӱ뾶W��Z��X��Y����X��Y��Ԫ�ش���ͬһ���ڣ�X��Z����ͬһ���壬��X��Y��Z��Ԫ�������ڱ��е����λ�ù�ϵΪ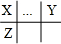 ��X��Y����ͬһ���壬�������������8��Zԭ�Ӻ��ڵ�����������X��Yԭ�Ӻ���������֮�ͣ���Y��������Ϊ8��Ϊ��Ԫ�أ�Zԭ��������ϵĵ�������Wԭ��������ϵĵ�������4������W����������Ϊ1��Z������������Ϊ4����XΪ̼Ԫ�ء�ZΪSiԪ�أ�W����������Ϊ1�����ڢ�A�壬ԭ�Ӱ뾶���ڹ裬��WΪNaԪ�أ��ݴ˽��
��X��Y����ͬһ���壬�������������8��Zԭ�Ӻ��ڵ�����������X��Yԭ�Ӻ���������֮�ͣ���Y��������Ϊ8��Ϊ��Ԫ�أ�Zԭ��������ϵĵ�������Wԭ��������ϵĵ�������4������W����������Ϊ1��Z������������Ϊ4����XΪ̼Ԫ�ء�ZΪSiԪ�أ�W����������Ϊ1�����ڢ�A�壬ԭ�Ӱ뾶���ڹ裬��WΪNaԪ�أ��ݴ˽��
��� �⣺X��Y��Z��W���Ƕ���������Ԫ�أ�ԭ�Ӱ뾶W��Z��X��Y����X��Y��Ԫ�ش���ͬһ���ڣ�X��Z����ͬһ���壬��X��Y��Z��Ԫ�������ڱ��е����λ�ù�ϵΪ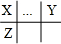 ��X��Y����ͬһ���壬�������������8��Zԭ�Ӻ��ڵ�����������X��Yԭ�Ӻ���������֮�ͣ���Y��������Ϊ8��Ϊ��Ԫ�أ�Zԭ��������ϵĵ�������Wԭ��������ϵĵ�������4������W����������Ϊ1��Z������������Ϊ4����XΪ̼Ԫ�ء�ZΪSiԪ�أ�W����������Ϊ1�����ڢ�A�壬ԭ�Ӱ뾶���ڹ裬��WΪNaԪ�أ�
��X��Y����ͬһ���壬�������������8��Zԭ�Ӻ��ڵ�����������X��Yԭ�Ӻ���������֮�ͣ���Y��������Ϊ8��Ϊ��Ԫ�أ�Zԭ��������ϵĵ�������Wԭ��������ϵĵ�������4������W����������Ϊ1��Z������������Ϊ4����XΪ̼Ԫ�ء�ZΪSiԪ�أ�W����������Ϊ1�����ڢ�A�壬ԭ�Ӱ뾶���ڹ裬��WΪNaԪ�أ�
��1����������ķ�����֪��XΪ̼��YΪ����ZΪ�裬WΪ�ƣ�
�ʴ�Ϊ��̼�������裻�ƣ�
��2��ZΪSiԪ�أ�ZԪ�������ڱ��е�λ���ǵ�3���� �ڢ�A�壬
�ʴ�Ϊ����3���� �ڢ�A�壻
��3��Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ������ڷǽ�����O��C��Si�������ǽ���Ԫ�أ�����������Ԫ���еķǽ����⻯����ȶ����ɴ�С��˳����H2O��CH4��SiH4��NaH��
�ʴ�Ϊ��H2O��CH4��SiH4��NaH��
��4��X��Y�γɵ���ԭ�ӷ���ΪCO2�����ĵ���ʽ��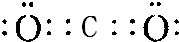 ��Y��W�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����Ϊ�������ƣ����ĵ���ʽ��
��Y��W�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����Ϊ�������ƣ����ĵ���ʽ��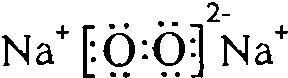 ��
��
�ʴ�Ϊ��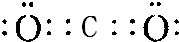 ��
��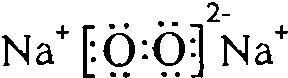 ��
��
��5��WΪ�ƣ�WԪ������������Ӧˮ����Ļ�ѧʽ��NaOH���볣���������������ﷴӦ�Ļ�ѧ����ʽΪNaOH+Al��OH��3=NaAlO2+2H2O��
�ʴ�Ϊ��NaOH��NaOH+Al��OH��3=NaAlO2+2H2O��
���� ���⿼��Ԫ���ƶϡ��۷е�Ƚϡ�����ˮ�⡢�����ṹ�ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���ؼ���ע�����ʽṹ����֪ʶ��������ã�

| A�� | Fe | B�� | Al��OH��3 | C�� | NO | D�� | H2SO3 |
| A�� | 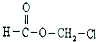 | B�� | 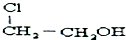 | C�� | CH2ClCHO | D�� | HOCH2CH2OH |
| A�� | ����֪ˮ�ķֽⷴӦ���ڷ��ȷ�Ӧ | |
| B�� | H2��O2��g����Ӧ�ų���������Ϊ�к��� | |
| C�� | 1mol H2��ȫȼ������Һ̬ˮ�ų�������С��24l.8kJ | |
| D�� | �Ͽ�1molH2O�Ļ�ѧ�����յ����������ڶ���lmolH2��0.5molO2�Ļ�ѧ�������յ������� |
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� ��25�棩 | Ka=1.77��10-4 | Ka=4.9��10-10 | Ka1=4.3��10-7 Ka2=5.6��10-11 |
| A�� | 2CN-+H2O+CO2=2HCN+CO32- | |
| B�� | �к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں��� | |
| C�� | ���ʵ���Ũ����ȵ�HCOONa��KCN��Һ�У�c��HCOOH��+c��HCOO-��=c��CN-��+c��HCN�� | |
| D�� | c��NH4+����ȵ�HCOONH4��Һ��NH4CN��Һ�У�c��NH4CN����c��HCOONH4�� |
| A�� | �����������л�����Ȳ�� | |
| B�� | ��ʹ��ˮ��ɫ��������ϩ����Ȳ�� | |
| C�� | ��Ȳ����������ԭ�Ӷ���ͬһֱ���� | |
| D�� | ����ʽΪC5H8������һ����Ȳ�� |
