��Ŀ����
�绯ѧԭ���ڹ�ҵ������������Ҫ�����ã���������ѧ֪ʶ�ش��й����⡣
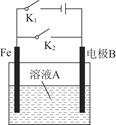
��1���õ��ķ�����������Һ����Ϊ��������о�������Ҫ��ʵ�����壬������ת��Ϊ�������ǵ�ⷨ�������������һ����Ҫ���ݡ���ͼ���ǵ������������ʵ��װ�ã�
����֪�����ķ�ӦΪ��x��1��S2��=Sx��S2����2xe�����������ĵ缫��Ӧʽ��________________________________________________________________________��
����Ӧת��x mol����ʱ���������������Ϊ____________����״���£���
�ڽ�Na2S��9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⡣��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ����___________________________________________________��
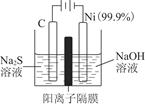
��2��MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪ______________________������Ǧ����Ϊ��Դ����ữ��MnSO4��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ_______________________________________________������������4 mol H��������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ________��MnO2�����۲���Ϊ________g��

��3����ͼ���װ�ÿ��Ƶþ��о�ˮ���õ�FeO42-��ʵ������У������������������Y������Һ������FeO42-��
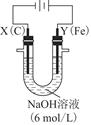
�ٵ������У�X������Һ��pH________���������С�����䡱����
�ڵ������У�Y�������ĵ缫��ӦΪFe��6e����8OH��=FeO42-��4H2O��________________________________________________________________________��
����X���ռ���672 mL���壬��Y���ռ���168 mL���壨��������Ϊ��״��ʱ�������������Y�缫�����缫����������________g��
��1���õ��ķ�����������Һ����Ϊ��������о�������Ҫ��ʵ�����壬������ת��Ϊ�������ǵ�ⷨ�������������һ����Ҫ���ݡ���ͼ���ǵ������������ʵ��װ�ã�

����֪�����ķ�ӦΪ��x��1��S2��=Sx��S2����2xe�����������ĵ缫��Ӧʽ��________________________________________________________________________��
����Ӧת��x mol����ʱ���������������Ϊ____________����״���£���
�ڽ�Na2S��9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⡣��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ����___________________________________________________��
��2��MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪ______________________������Ǧ����Ϊ��Դ����ữ��MnSO4��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ_______________________________________________������������4 mol H��������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ________��MnO2�����۲���Ϊ________g��

��3����ͼ���װ�ÿ��Ƶþ��о�ˮ���õ�FeO42-��ʵ������У������������������Y������Һ������FeO42-��

�ٵ������У�X������Һ��pH________���������С�����䡱����
�ڵ������У�Y�������ĵ缫��ӦΪFe��6e����8OH��=FeO42-��4H2O��________________________________________________________________________��
����X���ռ���672 mL���壬��Y���ռ���168 mL���壨��������Ϊ��״��ʱ�������������Y�缫�����缫����������________g��
��1����2H2O��2e��=2OH����H2������2H����2e��=H2������11.2x L
��2S2����O2��2H2O=2S����4OH��
��2��Mn2����2e����2H2O=MnO2��4H��
Pb��PbO2��2H2SO4=2PbSO4��2H2O
2 mol��87
��3������4OH����4e��=2H2O��O2����0.28
��2S2����O2��2H2O=2S����4OH��
��2��Mn2����2e����2H2O=MnO2��4H��
Pb��PbO2��2H2SO4=2PbSO4��2H2O
2 mol��87
��3������4OH����4e��=2H2O��O2����0.28
��1�����ʱ��ˮ�����H�������������õ��ӻ�ԭ��Ӧ������H2�����ݵ����غ��֪��x mol����ת�ƣ�����H2 0.5x mol��S2�����н�ǿ��ԭ�ԣ��ױ������е�������������������Һʱ��Ҫ��������������
��2����������Mn2��ʧ��������MnO2�������е���Ԫ����Դ��ˮ������H�����ٽ��缫����ʽ��ƽ���ɡ�
��3��ͼ��X���ĵ缫��ӦΪ2H����2e��=H2������2H2O��2e��=H2����2OH����������X������pH��������������672 mL����֪�õ�����Ϊ0.06 mol��Y����������Ϊ168 mL��ʧ������0.03 mol���ɵ�ʧ�����غ��֪��ʧ������Ϊ0.03 mol���ɵ缫��Ӧ��֪���ܽ�Ϊ0.005 mol����0.28 g��
��2����������Mn2��ʧ��������MnO2�������е���Ԫ����Դ��ˮ������H�����ٽ��缫����ʽ��ƽ���ɡ�
��3��ͼ��X���ĵ缫��ӦΪ2H����2e��=H2������2H2O��2e��=H2����2OH����������X������pH��������������672 mL����֪�õ�����Ϊ0.06 mol��Y����������Ϊ168 mL��ʧ������0.03 mol���ɵ�ʧ�����غ��֪��ʧ������Ϊ0.03 mol���ɵ缫��Ӧ��֪���ܽ�Ϊ0.005 mol����0.28 g��

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ

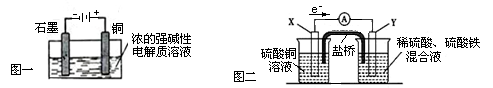
 2Li++H2��
2Li++H2��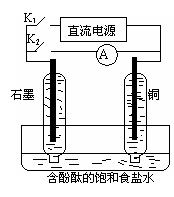
 Cl2��+H2��
Cl2��+H2��