��Ŀ����
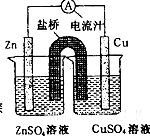
ij��ȤС�������ͼ��ʾ����ʵ��װ�á�ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�����һ��ʱ��Ͽ�K1���պ�K2�����ֵ�����ָ��ƫת�������й�������ȷ����
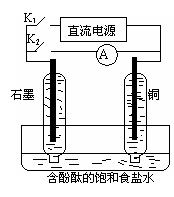

A���Ͽ�K2���պ�K1ʱ���ܷ�Ӧ�����ӷ���ʽΪ��2H+ + 2Cl- Cl2��+H2�� Cl2��+H2�� |
| B���Ͽ�K2���պ�K1ʱ��ʯī�缫������Һ��� |
| C���Ͽ�K1���պ�K2ʱ��ͭ�缫�ϵĵ缫��ӦΪ�� Cl2+2e-��2Cl- |
| D���Ͽ�K1���պ�K2ʱ��ʯī�缫������ |
D
���������A��ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�������ⱥ��ʳ��ˮ��ͭ���������������ʯī����������߷ų�������ͭ���������ұ߷ų���������Һ�Լ��ԣ�2H2O+ 2Cl-
 Cl2��+H2��+2OH-������ B���ұ�ͭ�缫������Һ��죬����C���Ͽ�K1���պ�K2�����ԭ��أ�Cl2+H2=2HCl������ʧ������������������������ʯī�缫�ϵĵ缫��ӦΪ�� Cl2+2e-��2Cl-������D��ʯī�缫����������ȷ��
Cl2��+H2��+2OH-������ B���ұ�ͭ�缫������Һ��죬����C���Ͽ�K1���պ�K2�����ԭ��أ�Cl2+H2=2HCl������ʧ������������������������ʯī�缫�ϵĵ缫��ӦΪ�� Cl2+2e-��2Cl-������D��ʯī�缫����������ȷ��
��ϰ��ϵ�д�
�����Ŀ
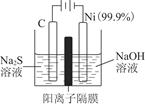

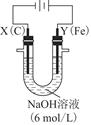
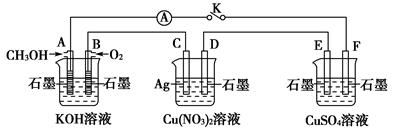
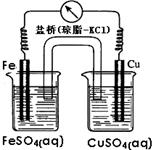
 Na2Sx���ش��������⣺
Na2Sx���ش��������⣺