��Ŀ����
3��ijǿ������ҺX�к�Ba2+��Al3+��SiO32-��NH4+��Fe2+��Fe3+��CO32-��SO42-��NO3-�е�һ�ֻ��������ӣ�ȡ��Һ��������ʵ�飬��ʵ������ת����
����������Ϣ���ش��������⣺
��1�����������У���ҺX�п϶����е��ǣ�Al3+��SO42-��NH4+��Fe2+�����ܿ϶����ǣ�Fe3+���Բ���ȷ���Ƿ���ڵ����ӣ�������ȡX��Һ��һ֧�Թ��У�ѡ�������Լ��е�һ�ּ���X��Һ�У���������Ϳ��жϣ�����Լ��ǣ��ݣ���ѡ���NaOH��Һ���ڷ�̪�Լ�����ʯ���Լ�����pH��ֽ����KSCN��Һ����KMnO4��Һ��
��2������D�Ļ�ѧʽΪ��NH3������E�Ļ�ѧʽΪ��Fe��OH��3������H�Ļ�ѧʽΪ��Al��OH��3��
��3��д����������з�����Ӧ�����ӷ���ʽBa2++SO42-=BaSO4����3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
���� ijǿ������ҺX������Һ�в��������������CO32-��SiO32-����Һ�����ᱵ��Һ��Ӧ���ɳ���C������Һ����SO42-�������ں���������ӷ�Ӧ��Ba2+����C��BaSO4��X�����ᱵ��Һ��Ӧͬʱ��������A��������������������Ӿ���ǿ�����ԣ��ɸ��������ӿ�֪��X��Һ�д���Fe2+����X��Һ������NO3-����A��NO����ҺB�м�������������Һʱ����������D����X��Һ�к���NH4+��D��NH3��������ҺB�д���Fe3+������E��Fe��OH��3��GΪFeCl3����ҺF��ͨ�����������̼���ɳ������ɸ��������ӿ�֪����X��Һ�д���Al3+������H��Al��OH��3����ҺI��NaHCO3������ȷ��X��Һ���Ƿ���Fe3+���ݴ˽��
��� �⣺ijǿ������ҺX������Һ�в��������������CO32-��SiO32-����Һ�����ᱵ��Һ��Ӧ���ɳ���C������Һ����SO42-�������ں���������ӷ�Ӧ��Ba2+����C��BaSO4��X�����ᱵ��Һ��Ӧͬʱ��������A��������������������Ӿ���ǿ�����ԣ��ɸ��������ӿ�֪��X��Һ�д���Fe2+����X��Һ������NO3-����A��NO����ҺB�м�������������Һʱ����������D����X��Һ�к���NH4+��D��NH3��������ҺB�д���Fe3+������E��Fe��OH��3��GΪFeCl3����ҺF��ͨ�����������̼���ɳ������ɸ��������ӿ�֪����X��Һ�д���Al3+������H��Al��OH��3����ҺI��NaHCO3������ȷ��X��Һ���Ƿ���Fe3+��
��1��������������֪����ҺX�п϶��������������е�SO42-��Al3+��NH4+��Fe2+������ȷ���Ƿ��е�����Fe3+��ȷ��������Fe3+�Ƿ���ڣ�ȡ����X�����Թ��У����뼸��KSCN��Һ����Һ����Ѫ��ɫ˵����Fe3+������Ѫ��ɫ֤������Fe3+�������Լ����ܼ���Fe3+��
�ʴ�Ϊ��Al3+��SO42-��NH4+��Fe2+��Fe3+���ݣ�
��2��������������֪������D�Ļ�ѧʽΪ��NH3������E�Ļ�ѧʽΪ��Fe��OH��3������H�Ļ�ѧʽΪ��Al��OH��3��
�ʴ�Ϊ��NH3��Fe��OH��3��Al��OH��3��
��3���������ǿ������Һ�м���������ᱵ�������Ӻ���������ӷ�Ӧ�������ᱵ�������������ӷ�ӦΪ��Ba2++SO42-=BaSO4�������������Ӿ��л�ԭ�ԣ�������������ԣ����������ӱ����������������������ӣ����ᱻ��ԭ��һ���������������ӷ�ӦΪ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
�ʴ�Ϊ��Ba2++SO42-=BaSO4����3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
���� ���⿼�����Ӽ��顢�����ƶϣ��ؼ��Ǹ���ʵ�������жϴ��ڵ����ӣ���Ҫѧ��������Ԫ�ػ�����֪ʶ���Ѷ��еȣ�

| A�� | H2O�ķе�Ƚϸߣ�������ˮ���Ӽ���������ԭ�� | |
| B�� | ��ϡ��������ķǽ���Ԫ�ض������ɲ�ͬ��̬�ĺ����� | |
| C�� | ����A��ijԪ�ص�ԭ������Ϊm����ͬ���ڢ�A��Ԫ�ص�ԭ����������Ϊm+11 | |
| D�� | ���ڷ��Ӽ��������Ȼ�ѧ�����ö࣬���Ըɱ��������ף���CO2����ֽ�ȴ���� |
| A�� | ��M����������ϼ�Ϊ+4��������Ԫ�ض��Ƿǽ���Ԫ�� | |
| B�� | HnJOmΪǿ�ᣬ��G��λ�ڢ�A���Ժ�Ļ��÷ǽ���Ԫ�� | |
| C�� | ��T����ͻ��ϼ�Ϊ-3����J����������ϼ�Ϊ+7 | |
| D�� | ��R��M������������ˮ�����Ϊ���R��OH��n�ļ���һ����M��OH��n+1�ļ���ǿ |
| A�� | ��λ�����ڱ��ĵ������ڡ���IA�� | |
| B�� | ̼������ȷֽ�Ϊ����墨Ͷ�����̼ | |
| C�� | 卑�������������ˮ | |
| D�� | �Ȼ�������ӻ����� |
| A�� | �������ۼ���AgNO3��Һ�� | |
| B�� | ����Na2O2��ĩ����CuSO4��Һ�� | |
| C�� | ����BaSO4��ĩ���뱥��Na2CO3��Һ�� | |
| D�� | ����Al2��SO4��3��ĩ����Ba��OH��2��Һ�� |
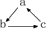 ��ϵ�ת�����ǣ���������ʾһ����ɣ���������
��ϵ�ת�����ǣ���������ʾһ����ɣ���������| ѡ�� | A | B | C | D |
| a | SiO2 | NaOH | HNO3 | Cu |
| b | Na2SiO3 | Na2CO3 | NO | CuSO4 |
| c | H2SiO3 | NaHCO3 | NO2 | Cu��OH��2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
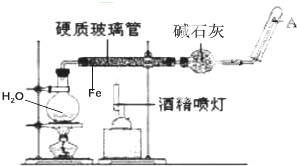 ��ͼ���ڸ����£�Fe��ˮ�����ķ�Ӧʵ�飮
��ͼ���ڸ����£�Fe��ˮ�����ķ�Ӧʵ�飮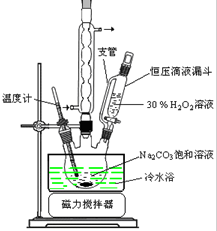 2Na2CO3•3H2O2��һ�����͵���ϵƯ����ijʵ����ȤС�����������ʵ�飮
2Na2CO3•3H2O2��һ�����͵���ϵƯ����ijʵ����ȤС�����������ʵ�飮