��Ŀ����
��7�֣���ͼ��ʾ�����رշ���Kʱ������г���1.5 mol A��3.5 mol B�������г���3 mol A��7 mol B����ʼʱ���ס��������ΪVL������ͬ�¶Ⱥ��д������ڵ������£��������и��Է������з�Ӧ��3A(g)��2B(g) 2C(g)��4D(g)����H<0�ﵽƽ�⣨��ʱ��V(��)=1.21V L����ش�
2C(g)��4D(g)����H<0�ﵽƽ�⣨��ʱ��V(��)=1.21V L����ش�

��1������B��ת����Ϊ ��
��2������D������C�����ʵ����Ƚϣ� �����ȡ�����ǰ�ߴ������ߴ���
��3����K����һ��ʱ�����´�ƽ�⣨��ʱ���ҵ����Ϊ ���ú�V�Ĵ���ʽ��ʾ����ͨ��������������Բ��ƣ���
 2C(g)��4D(g)����H<0�ﵽƽ�⣨��ʱ��V(��)=1.21V L����ش�
2C(g)��4D(g)����H<0�ﵽƽ�⣨��ʱ��V(��)=1.21V L����ش�
��1������B��ת����Ϊ ��
��2������D������C�����ʵ����Ƚϣ� �����ȡ�����ǰ�ߴ������ߴ���
��3����K����һ��ʱ�����´�ƽ�⣨��ʱ���ҵ����Ϊ ���ú�V�Ĵ���ʽ��ʾ����ͨ��������������Բ��ƣ���
��1��60�G��2�֣�
��2��ǰ�ߴ�2�֣�
��3��0.815V��3�֣�
���������
��1�����÷���ʽ���м��㣺
3A(g)��2B(g)
 2C(g)��4D(g)
2C(g)��4D(g)n������ 3mol 7mol
��n 9a mol 6a mol 6a mol 12a mol
n��ĩ��(3-9a) mol (7-6a)mol 6a mol 12a mol
���ԣ�(3-9a+7-6a+6a+12a)/10=12.1��a="0.7" ����B��ת����Ϊ 6a/7=60%
��2��ת���ݵ�Чƽ���ԭ�����з�Ӧ�����Ϊ���е������������ҵ�ƽ��״̬�и����ʵ���С�ڼ��������������м�ѹʹƽ�����ƶ�����������C�����ʵ����ȼ���D�����ʵ���С��
��3�����һ�Ϻ����ҵ�ƽ��״̬��Ϊ��Чƽ�⡣��60%��ת�����������ʣ���������������ҵ����Ϊ0.815V��
�����������Գ����Ļ�����ģ�ͣ����ԼĻ�ѧ���㣬���һ����Ե�Чƽ��������⡣��

��ϰ��ϵ�д�
�����Ŀ
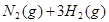
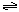
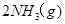
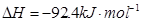 ����
����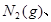 3mol
3mol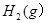 ����һ�ݻ�Ϊ2L���ܱ������У���500���½��з�Ӧ��10min
����һ�ݻ�Ϊ2L���ܱ������У���500���½��з�Ӧ��10min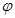 ������˵������ȷ���ǣ� ��
������˵������ȷ���ǣ� ��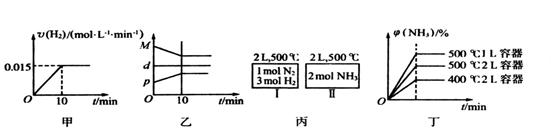
 pC(��)��ƽ������������С��ԭ����
pC(��)��ƽ������������С��ԭ����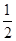 �����ﵽ��ƽ��ʱ��C��Ũ��Ϊԭ����1.9������ѹ�������б����¶Ȳ��䣬��Ӧ����ʽ������ϵ����ϵ�ǣ� ��
�����ﵽ��ƽ��ʱ��C��Ũ��Ϊԭ����1.9������ѹ�������б����¶Ȳ��䣬��Ӧ����ʽ������ϵ����ϵ�ǣ� �� 2C(g)������ѹǿ��ƽ��������ƽ����Է���������С
2C(g)������ѹǿ��ƽ��������ƽ����Է���������С nC(g)�ķ�Ӧ��ϵ�У�C�İٷֺ���(c%)��ʱ��t����������ͼ��ʾ�����������ȷ����
nC(g)�ķ�Ӧ��ϵ�У�C�İٷֺ���(c%)��ʱ��t����������ͼ��ʾ�����������ȷ����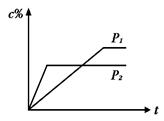
 2B(g) ��2A(g)
2B(g) ��2A(g) HClO��OH�������д�ʩ�������Ư��Ч�����( )
HClO��OH�������д�ʩ�������Ư��Ч�����( ) bB(g)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ�µ�ƽ��ʱ��B��Ũ����ԭ����60������ ( )
bB(g)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ�µ�ƽ��ʱ��B��Ũ����ԭ����60������ ( ) x C(g)��2minʱ��Ӧ�ﵽƽ��״̬(�¶Ȳ���)��ʣ��1.8molB�������C��Ũ��Ϊ0.4mol/L���ݴ���д���¿հף�
x C(g)��2minʱ��Ӧ�ﵽƽ��״̬(�¶Ȳ���)��ʣ��1.8molB�������C��Ũ��Ϊ0.4mol/L���ݴ���д���¿հף�