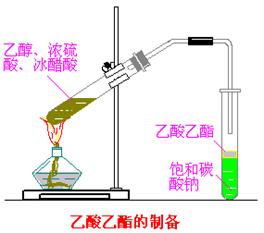
��Ŀ����
��8�֣���̼���ƣ�Na2CO3��3H2O2�����й���˫��ˮ���׳ƣ�������Ӧ����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������У���̼���Ƶ�ij������������ͼ��ʾ����֪��2Na2CO3+3H2O2=2Na2CO3��3H2O2 ��H<0���ش��������⣺
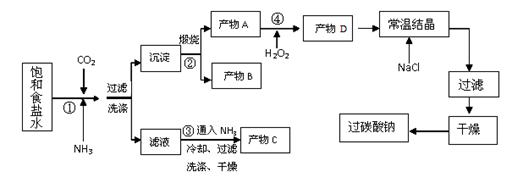
��1���������ʿ�ʹ��̼���ƽϿ�ʧЧ����(�����) ��
��2����֪����C��һ�ֳ��õ��ʣ����仯ѧʽΪ ��
��Ӧ�ٵ��ܷ�Ӧ����ʽΪ ��
��3�������������п�ѭ��ʹ�õ������� ��
��4��������̼���Ƶ���������©��һ����������ò�Ʒ����ƫ�ͣ��ò������������� ����

��1���������ʿ�ʹ��̼���ƽϿ�ʧЧ����(�����) ��
| A��FeCl3��Һ | B��H2S | C��ϡ���� | D��NaHCO3��Һ |
��Ӧ�ٵ��ܷ�Ӧ����ʽΪ ��
��3�������������п�ѭ��ʹ�õ������� ��
��4��������̼���Ƶ���������©��һ����������ò�Ʒ����ƫ�ͣ��ò������������� ����
����8�֣�
��1��C��3�֣�
��2��NH4Cl ��1�֣� NaCl+CO2+H2O+NH3=NaHCO3 ��+NH4Cl��2�֣�
��3��CO2��1�֣�
��4�������ϴ�ӣ�1�֣�
��1��C��3�֣�
��2��NH4Cl ��1�֣� NaCl+CO2+H2O+NH3=NaHCO3 ��+NH4Cl��2�֣�
��3��CO2��1�֣�
��4�������ϴ�ӣ�1�֣�
�����������1������̼���ƻ�˫��ˮ�Ļ�ѧ���ʷ�����
��2������˫��ˮ�IJ��ȶ��Է�������3�����ݻ�ѧ����ʽ�ļ����ʽ�������Լ��������������ļ��㹫ʽ������𡣢ٷ����ķ�Ӧ����ʽΪ��NaCl+CO2+H2O+NH3=NaHCO3 ��+NH4Cl����CΪNH4Cl��
��3������������Ӧ�����̿��Եó����������п�ѭ��ʹ�õ�������CO2��
��4��������û�о����ϴ�ӣ���Ʒ���Ȼ�ƫ�͡�
�����𣺣�1������̼���Ʊ��ʵ�ԭ������̼���ƻ�˫��ˮ��Ӧ�ˣ�A������FeCl3��Һ��ʹ˫��ˮ�ֽ�����ʣ�B����H2S����˫��ˮ��Ӧ�����ʣ�C����ϡ���ᣬ��̼���Ʒ�Ӧ�����ʣ� D����NaHCO3��Һ����̼���ƻ�˫��ˮ����Ӧ�������ʣ���ѡA��B��C��
��2��
���������⿼����˫��ˮ��̼���ƵĻ�ѧ���ʼ��й���Һ����ѧ����ʽ�ļ��㣬ע�ؿ���ѧ���ķ��������ͻ�������������

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ