��Ŀ����
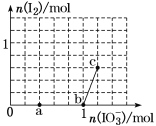
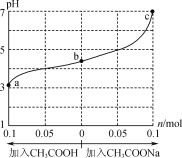
����Ŀ����25��ʱ����1.0Lwmol��L-1CH3COOH��Һ��0.1molNaOH�����ϣ���ַ�Ӧ��Ȼ������Һ�м���CH3COOH��CH3COONa����(����������¶ȱ仯)����ҺpH�ı仯��ͼ��ʾ������������ȷ���ǣ� ��
A.b����Һ�У�c(Na+)<c(CH3COOH)+c(CH3COO-)
B.��b�㵽a��Ĺ����У�c(Na+)�п��ܴ���c(CH3COO-)
C.a��b��c��Ӧ�Ļ��Һ�У�ˮ�ĵ���̶��ɴ�С��˳����c>a>b
D.25��ʱ��Ka(CH3COOH)=![]() mol��L-1
mol��L-1
���𰸡�AD
��������
1.0L w mol��L-1 CH3COOH��Һ��0.1mol NaOH�����ϣ���Ϻ���Һ��pH��5�������ԣ�˵�������������Һ������ΪCH3COOH��CH3COONa���Ӵ���ʱ����������࣬ʹ��Һ������ǿ����CH3COONaʱ��CH3COONa����ˮ���Լ��ԣ��൱�ڼӼʹ��Һ���Լ��������մﵽ���ԡ�
A��1.0L w mol��L-1 CH3COOH��Һ��0.1mol NaOH�����ϣ���Ϻ���Һ��pH��5�������ԣ�˵�������������Һ������ΪCH3COOH��CH3COONa��b����Һ�У�c(Na+)<c(CH3COOH)+c(CH3COO-)����A��ȷ��
B����b�㵽a��Ĺ����У�һֱ�Ǵ��������c(Na+)С��c(CH3COO-)����B����
C����Һ���������������ӻ����������ӵ�Ũ��Խ��ˮ�ĵ���̶�ԽС��a��b��c������Һ��������Ũ�����μ�С��ˮ�ĵ���̶���������ˮ�ĵ���̶��ɴ�С��˳�����c��b��a����C����
D������ͼ���֪��c����Һ��pH=7��c��H����=c��OH����=10-7mol��L��1�����ݵ���غ��֪��c��CH3COO����=c��Na����=0.2mol��L��1��c��CH3COOH��=w+0.1-0.2=w-0.1����CH3COOH�ĵ���ƽ�ⳣ��Ka=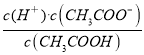 =
=![]() mol��L-1����D��ȷ��
mol��L-1����D��ȷ��
��ѡAD��