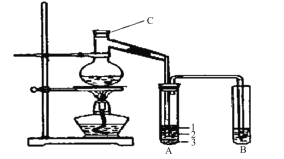
��Ŀ����
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���

��1������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳���ǣ�дԪ�ط��ţ�_________________________��
��2��Ԫ��Y����Ԫ���γɵ�һ������YH4+��д�������ĵ���ʽ________(��Ԫ�ط��ű�ʾ)��
��3��TԪ�صļ������ӵĽṹʾ��ͼ��______________________��
��4��֤�������ӵĻ�ԭ�Ա�Z-ǿ�����ӷ���ʽ��____________________.
��5��Ԫ��Z�ĵ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ_______________________ ��
��6����9g����X������������ȼ�գ���������ͨ��1L 1mol��L-1NaOH��Һ�У���ȫ���պ���Һ�ڼ�ѹ�������������ɣ��õ������ᾧˮ�Ĺ�������Ϊ____________g��
���𰸡���1��S Cl C N (2��)��
��2��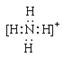 ��1�֣���
��1�֣���
��3�� ��1�֣���
��1�֣���
��4��C12+ S2-=2Cl-+S����2�֣���
��5��Cl2+ 2NaOH="NaCl+" NaClO +H2O (2��)��
��6��68.5g��2�֣���
�����������������������Ԫ��X�����������Ǵ�����2������X��CԪ�أ�Y��������˫ԭ�ӷ��ӣ��⻯���ˮ��Һ�Լ��ԣ���Y��NԪ�أ�T�����������ǵ��Ӳ�����2������T��SԪ�أ�ZԪ������ϼ���+7�ۣ���Z��ClԪ�ء�
��1��ԭ�Ӻ�����Ӳ���Խ�࣬ԭ�Ӱ뾶Խ��ԭ�Ӻ�����Ӳ�����ͬʱ��ԭ������ԽС��ԭ�Ӱ뾶Խ����������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����S�� Cl ��C ��N��
��2��NH4+����ʽ��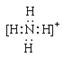 ��
��
��3��TԪ����S����������ӵĽṹʾ��ͼ��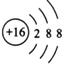 ��
��
��4��Ԫ�صķǽ�����Խǿ����������ӵĻ�ԭ�Ծ�Խ����Ԫ�صķǽ�����Cl��S�����Ի�ԭ��Cl-��S2-��֤���ķ�Ӧ��C12+ S2-=2Cl-+S����
��5��Ԫ��Z�ĵ���Cl2������NaOH��Һ������Ӧ������NaCl��NaClO��ˮ������������������Һ��Ӧ�Ļ�ѧ����ʽΪCl2+ 2NaOH="NaCl+" NaClO +H2O��
��6��9gC�����ʵ�����n(C)=9g��12g/mol=0.75mol������CԪ���غ��֪�������CO2�����ʵ�����0��75mol��1L 1mol��L-1NaOH��Һ���е����ʵ����ʵ�����n(NaOH)=c��V=1mol/L��1L=1mol����CO2ͨ�뵽NaOH��Һ��������Ӧ����̼���ơ�̼�����ƣ�����C�غ��֪n(Na2CO3)+n(NaHCO3��=0.75mol������NaԪ���غ�ɵ�2n(Na2CO3)+n(NaHCO3��=1mol�����n(Na2CO3)=0.25mol��n(NaHCO3��=0.5mol����ȫ���պ���Һ�ڼ�ѹ�������������ɣ��õ������ᾧˮ�Ĺ�������Ϊm=0.25mol��106g/mol+0.5mol��84g/mol=26.5g+42g=68.5g��