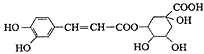��Ŀ����
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�飬�������к͵ζ���������ԭ�ζ��ͳ����ζ���
��.����к͵ζ�������֪ijNaOH�����к���NaCl���ʣ��ñ�����ⶨ������NaOH�������������������²���ʵ�飺
�ٳ���һ����������Ʒ����ˮ�������Һ����ȷ��ȡһ�����������Һ����ƿ�У�
�۵μӼ��η�̪��Һ�����ñ�����ζ����ظ��������Σ���ش��������⣺
��1���ζ���ʹ��ʱ�ĵ�һ��������____________________________��
��2������50.00mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10����������ڵ����������ţ�_____���٣�10mL���ڣ�40mL����<10mL����>40mL����
��3�����ζ����յ�ʱ���Ӷ��������ɴ˲��ʽ����NaOH�ĺ�����__________������ƫ������ƫС����������������
��.����֪Ũ�ȵĸ��������Һ�ⶨδ֪������Һ��Ũ�ȣ�ȡһ�����δ֪Ũ�ȵIJ�����Һ����ƿ�У���������ϡ���ᣬ����֪Ũ�ȵĸ��������Һ�ζ���
��1��������Ӧ�����ӷ���ʽΪ��__________________________________
��2���ζ�ʱ��KMnO4��ҺӦװ��ͼ��_________���������������������ζ����У��ζ��յ�ʱ�ζ�������________________________________��

��3������������������ⶨ���ƫ�ߵ���_______________________________��
a.װ��Һ�ĵζ���������ˮϴ��δ�ñ�Һ��ϴ
b.������ƿʱ������ƿ����Һ����
c.���ڵζ������в��������α�Һ������ƿ��
d.��ƿ��δ֪Һ��ϴ
e.�ζ�ǰ������ˮ��ϴ��ƿ
���𰸡���© �� ƫС 2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O �� ���������һ��KMnO4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ acd
��������
��(1)�ζ�����ʹ��ǰ��Ҫ����Ƿ�©Һ��
(2)�ζ��ܵ�0�̶����Ϸ���10mL�̶����·�����40mL�п̶ȵ���Һ������ζ���50mL�̶�������Һ�壻
(3)�ζ����յ�ʱ���Ӷ����������ı�Һ���ƫС���ݴ˷������
��(1)�����������������������ᷢ��������ԭ��Ӧ����Mn2+��CO2��H2O���ݴ���д��Ӧ�ķ���ʽ��
(2)���������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ��ζ�����ǰ��ҺΪ��ɫ���ζ�����ʱ��Һ��Ϊdz��ɫ���ݴ��жϵζ��յ㣻
(3)���ݲ���������c(����)=![]() ��Ӱ�������
��Ӱ�������
I��(1)�ζ������ܻ���������ʹ��ǰ�����©���ʴ�Ϊ����©��
(2)����50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�������ζ��ܵ�0�̶����Ϸ���10mL�̶����·�����40mL�п̶ȵ���Һ������ζ���50mL�̶�������Һ�壬��˹��ڵ�Һ�������(50.00mL-10.00mL)=40.00mL���ʢ���ȷ���ʴ�Ϊ���ܣ�
(3)���ζ����յ�ʱ���Ӷ����������ı�Һ���ƫС���������NaOH�����ʵ���ƫС���ɴ˲��ʽ����NaOH�ĺ�����ƫС���ʴ�Ϊ��ƫС��
��(1)�����������ᷴӦ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O���ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
(2)��Ϊ��ʽ�ζ��ܣ���Ϊ��ʽ�ζ��ܣ����Ը��������Һ����ǿ�����ԣ�Ӧ������ʽ�ζ���ʢ�ţ������Ը��������Һ�ζ����ᣬ�ζ��յ�����Ϊ�����������һ��KMnO4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ���ף����������һ��KMnO4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
(3)a��װ��Һ�ĵζ���������ˮϴ��δ�ñ�Һ��ϴ�����±�ҺŨ�ȼ�С���ζ����������ı�Һ���ƫ�ⶨ���ƫ�ߣ���a��ȷ��b��������ƿʱ������ƿ����Һ�������������ı�Һ���ƫС���ⶨ���ƫ�ͣ���b����c�����ڵζ������в��������α�Һ������ƿ�⣬�������ı�Һ���ƫ�ⶨ���ƫ�ߣ���c��ȷ��d����ƿ��δ֪Һ��ϴ�����´���Һ���ƫ�ζ����������ı�Һ���ƫ�ⶨ���ƫ�ߣ���d��ȷ��e���ζ�ǰ������ˮ��ϴ��ƿ����Ӱ���Һ�������Ӱ��ζ��������e���ʴ�Ϊ��acd��