��Ŀ����
ijУ�о���ѧϰС�����ʵ��̽��Ԫ�������ɡ��ס�����ͬѧ���������ͼ��ʾʵ�顣
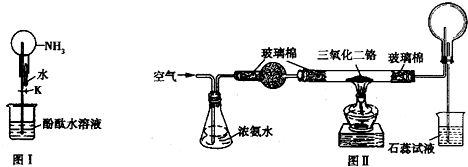
(1)��ͬѧ�������ͼ1װ�ã�����Ԫ�ص���ۺ�������������֤ͬ����Ԫ�طǽ�����ǿ���ıȽϣ���ͬѧ���ʵ���������___________�� д��ѡ�����ʵ����ƣ�A___________��B__________��C___________�� ��Ӧ�����ӷ���ʽΪ____________��______________��
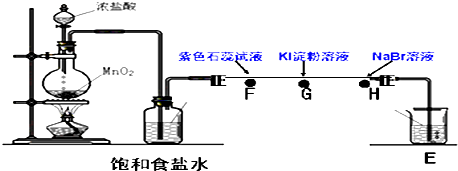
(2)��ͬѧ�������ͼ2װ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ���ֽ����֪������Ũ�������������ܷ�Ӧ������������ͬѧ��ʵ��ԭ����____________________�� д��B�������ӷ���ʽ��_________________��
(2)��ͬѧ�������ͼ2װ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ���ֽ����֪������Ũ�������������ܷ�Ӧ������������ͬѧ��ʵ��ԭ����____________________�� д��B�������ӷ���ʽ��_________________��
(1)ǿ�������� ��ϡ���� ��̼��� ����������Һ ��CaCO3+2H+ =Ca2+ +2CO2��+H2O ��
SiO32- +2CO2+ 2H2O=H2SiO3��+2HCO3-
(2)ǿ�����������������������õķǽ��������û��ϲ����õķǽ������� ��2I-+Cl2=2Cl-+I2
SiO32- +2CO2+ 2H2O=H2SiO3��+2HCO3-
(2)ǿ�����������������������õķǽ��������û��ϲ����õķǽ������� ��2I-+Cl2=2Cl-+I2

��ϰ��ϵ�д�
�����Ŀ
�����к��е�Ԫ�أ�ijУ�о���ѧϰС�����������ʵ�鲽������ȡ�⣺
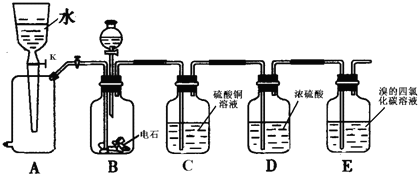
��ͨ�����������ڽ������ճɻң�����м�ˮ���裻�ۼ�CC14���ܹ��ˣ����÷�Һ©����Һ��
�����IJ���˳��Ϊ��������
��ͨ�����������ڽ������ճɻң�����м�ˮ���裻�ۼ�CC14���ܹ��ˣ����÷�Һ©����Һ��
�����IJ���˳��Ϊ��������
| A���١��ۡ��ݡ��ڡ��� | B���ڡ��١��ۡ��ܡ��� | C���ڡ��ܡ��١��ۡ��� | D���ۡ��١��ڡ��ݡ��� |
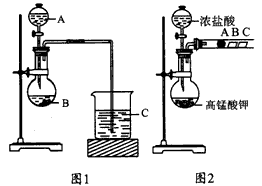
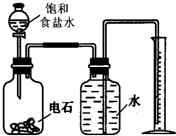 ��֪��ʯ�к��е�������ˮ��Ӧ������H2S��PH3���壬ijУ�о���ѧϰС�����ʵ��ⶨ��ʯ��Ʒ�Ĵ��ȣ��������й����ϵ�֪��H2S��PH3������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ�
��֪��ʯ�к��е�������ˮ��Ӧ������H2S��PH3���壬ijУ�о���ѧϰС�����ʵ��ⶨ��ʯ��Ʒ�Ĵ��ȣ��������й����ϵ�֪��H2S��PH3������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ�