��Ŀ����
 ��֪��ʯ�к��е�������ˮ��Ӧ������H2S��PH3���壬ijУ�о���ѧϰС�����ʵ��ⶨ��ʯ��Ʒ�Ĵ��ȣ��������й����ϵ�֪��H2S��PH3������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ�
��֪��ʯ�к��е�������ˮ��Ӧ������H2S��PH3���壬ijУ�о���ѧϰС�����ʵ��ⶨ��ʯ��Ʒ�Ĵ��ȣ��������й����ϵ�֪��H2S��PH3������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��ǣ�H2S+CuSO4�TCuS��+H2SO4
19PH3+56CuSO4+44H2O�T11H3PO4+56H2SO4+32Cu��+8Cu3P��
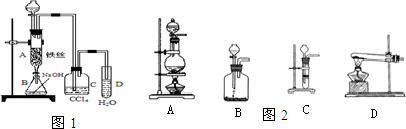
��1������ͬѧ�����ͼװ�òⶨ��ʯ��Ʒ�Ĵ��ȣ�����գ�
��д��ʵ����������Ȳ�Ļ�ѧ��Ӧ����ʽ��
CaC2+2H2O��Ca��OH��2+C2H2��
CaC2+2H2O��Ca��OH��2+C2H2��
���ڵ�ʯ��ַ�Ӧ���ڶ���֮ǰӦ
b c
b c
������ţ���a������������b��������Ͳ����ƿ��Һ����ƽ��c������������ȴ�����£�
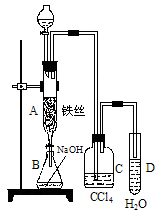
��2������ͬѧ��Ϊ����ͬѧ��Ƶ�ʵ��ⶨ�����ȷ�Ȳ��ߣ�����ʹ����ͼװ�ý���ʵ�飺

�Իش��������⣺
��E�з�����Ӧ�Ļ�ѧ����ʽΪ
CH��CH+2Br2��CHBr2CHBr2
CH��CH+2Br2��CHBr2CHBr2
����ʵ������ֱ�Ӳⶨ��������
a c
a c
��a����ʯ��Ʒ������ b��װ��C������ c��װ��E������ d��ʵ��ʱ�����º�ѹǿ
��ʵ��ʱ����B�г�ַ�Ӧ��Ӧ����ʹA������Ŀ�����������B�У���Ŀ����
ʹװ�������ɵ���Ȳ��E��������Ȼ�̼��Һ�������
ʹװ�������ɵ���Ȳ��E��������Ȼ�̼��Һ�������
����������1����ʯ��ˮ��Ӧ����Ca��OH��2��C2H2������ˮ������ʱӦ��������ȴ�����£��ҵ�����Ͳ����ƿ��Һ����ƽ��װ����ѹǿ�ʹ���ѹ��ȣ�
��2������ͬѧ��ʵ��Ŀ�����õ�ʯ��ˮ��Ӧ������Ȳ��������ͭ��ȥ���⡢��������ʣ��������������Ȼ�̼��Һ������Ȳ������������Ȼ�̼���������أ���ȷ����ʯ��̼���Ƶ�������Ϊ��֤���屻������գ����ÿ�����װ���ڵ�������ȫ�ų���
��2������ͬѧ��ʵ��Ŀ�����õ�ʯ��ˮ��Ӧ������Ȳ��������ͭ��ȥ���⡢��������ʣ��������������Ȼ�̼��Һ������Ȳ������������Ȼ�̼���������أ���ȷ����ʯ��̼���Ƶ�������Ϊ��֤���屻������գ����ÿ�����װ���ڵ�������ȫ�ų���
����⣺��1���ٵ�ʯ��ˮ��Ӧ����Ca��OH��2��C2H2����Ӧ�ķ���ʽΪCaC2+2H2O��Ca��OH��2+C2H2����
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+C2H2����
������ˮ������ʱӦ��������ȴ�����£��ҵ�����Ͳ����ƿ��Һ����ƽ��װ����ѹǿ�ʹ���ѹ��ȣ�
�ʴ�Ϊ��b c��
��2����E�з�����ӦΪ��Ȳ����ļӳɷ�Ӧ������ʽΪCH��CH+2Br2��CHBr2CHBr2��
�ʴ�Ϊ��CH��CH+2Br2��CHBr2CHBr2��
��Ϊ�ⶨ��ʯ��Ʒ�ĺ�����Ӧ������ʯ��Ʒ��������װ��E�����أ�����������Ȼ�̼���������أ���ȷ����ʯ��̼���Ƶ�������
�ʴ�Ϊ��a c��
��Ϊ��֤���屻������գ����ÿ�����װ���ڵ�������ȫ�ų�����B�г�ַ�Ӧ��Ӧ����ʹA������Ŀ�����������B�У�
�ʴ�Ϊ��ʹװ�������ɵ���Ȳ��E��������Ȼ�̼��Һ������գ�
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+C2H2����
������ˮ������ʱӦ��������ȴ�����£��ҵ�����Ͳ����ƿ��Һ����ƽ��װ����ѹǿ�ʹ���ѹ��ȣ�
�ʴ�Ϊ��b c��
��2����E�з�����ӦΪ��Ȳ����ļӳɷ�Ӧ������ʽΪCH��CH+2Br2��CHBr2CHBr2��
�ʴ�Ϊ��CH��CH+2Br2��CHBr2CHBr2��
��Ϊ�ⶨ��ʯ��Ʒ�ĺ�����Ӧ������ʯ��Ʒ��������װ��E�����أ�����������Ȼ�̼���������أ���ȷ����ʯ��̼���Ƶ�������
�ʴ�Ϊ��a c��
��Ϊ��֤���屻������գ����ÿ�����װ���ڵ�������ȫ�ų�����B�г�ַ�Ӧ��Ӧ����ʹA������Ŀ�����������B�У�
�ʴ�Ϊ��ʹװ�������ɵ���Ȳ��E��������Ȼ�̼��Һ������գ�
���������⿼�����ʵĺ����ⶨ��Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬�Ѷ��еȣ�ע�����ʵ���ԭ����ʵ�鷽�������ۣ�

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ
| |||||||||||||||

 ��
��