��Ŀ����
ά����C�ֳƿ���Ѫ�ᣬ�㷺������ˮ�����߲��У�������Դ��ά���أ����岻�������ϳɣ������ʳ������ȡ���仯ѧʽΪC6H8O6����Է�����Ϊ176.1�����ڷ����е�ϩ���������л�ԭ�ԣ��ܱ�I2�����������ɶ�ͪ�����뷴ӦΪ��
C6H8O6��C6H6O6��2H����2e jy��0.18V
��ˣ����Բ��õ������ⶨά����CҩƬ�п���Ѫ��ĺ���������ʵ�鲽�輰������£�
��1��ȷ��ȡ0.01667mol/L��K2Cr2O7����Һ10.00mL�ڵ���ƿ�У���3mol/L H2SO4��Һ10mL��10% KI��Һ10mL������ƿ�����������÷�Ӧ5min������100mLˮϡ�ͣ���Na2S2O3����Һ�ζ�������ɫʱ������2mL������Һ�������ζ�����Һ����ɫ��Ϊ����ɫ��ƽ������ʵ�飬����Na2S2O3����Һƽ�����Ϊ19.76mL��
��2��ȷ��ȡ����Na2S2O3����Һ10.00mL��ƿ�У���ˮ50mL��������Һ2mL����I2����Һ�ζ�����ɫ��30s���ʡ�ƽ������ʵ�飬����I2����Һƽ�����Ϊ10.15mL��
��3��ȷ��ȡ0.2205g��ά����C��ĩ��ά����CҩƬ��ϸ���ã���ƿ�У�������й�����ȴ������ˮ100mL��2 mol/L HAc��Һ10mL��������Һ2mL��������I2����Һ�ζ�����ɫ��30s���ʣ�����12.50mL��
��4���ظ��������裨3������ȡά����C��ĩ0.2176g������I2����ҺΪ12.36mL����ȡά����C��ĩ0.2332g������I2����ҺΪ13.21mL��
��������ʵ�����������ά����CҩƬ����������Ѫ�������������
C6H8O6��C6H6O6��2H����2e jy��0.18V
��ˣ����Բ��õ������ⶨά����CҩƬ�п���Ѫ��ĺ���������ʵ�鲽�輰������£�
��1��ȷ��ȡ0.01667mol/L��K2Cr2O7����Һ10.00mL�ڵ���ƿ�У���3mol/L H2SO4��Һ10mL��10% KI��Һ10mL������ƿ�����������÷�Ӧ5min������100mLˮϡ�ͣ���Na2S2O3����Һ�ζ�������ɫʱ������2mL������Һ�������ζ�����Һ����ɫ��Ϊ����ɫ��ƽ������ʵ�飬����Na2S2O3����Һƽ�����Ϊ19.76mL��
��2��ȷ��ȡ����Na2S2O3����Һ10.00mL��ƿ�У���ˮ50mL��������Һ2mL����I2����Һ�ζ�����ɫ��30s���ʡ�ƽ������ʵ�飬����I2����Һƽ�����Ϊ10.15mL��
��3��ȷ��ȡ0.2205g��ά����C��ĩ��ά����CҩƬ��ϸ���ã���ƿ�У�������й�����ȴ������ˮ100mL��2 mol/L HAc��Һ10mL��������Һ2mL��������I2����Һ�ζ�����ɫ��30s���ʣ�����12.50mL��
��4���ظ��������裨3������ȡά����C��ĩ0.2176g������I2����ҺΪ12.36mL����ȡά����C��ĩ0.2332g������I2����ҺΪ13.21mL��
��������ʵ�����������ά����CҩƬ����������Ѫ�������������
������̣��ȼ����Na2S2O3����Һ��Ũ�ȣ�Ȼ����Na2S2O3����Һ�궨I2����Һ��Ũ�ȣ��ٸ���I2����Һ��Ũ�ȼ��㿹��Ѫ�������������
��������Na2S2O3����Һ��Ũ��Ϊ0.05062 mol/L����4�֣�
I2����Һ��Ũ��Ϊ0.02494 mol/L����4�֣�
����Ѫ�����������ƽ��ֵΪ0.2491����4�֣�
��������Na2S2O3����Һ��Ũ��Ϊ0.05062 mol/L����4�֣�
I2����Һ��Ũ��Ϊ0.02494 mol/L����4�֣�
����Ѫ�����������ƽ��ֵΪ0.2491����4�֣�
�������漰�����з�Ӧ��Ϊ������ԭ��Ӧ����˵ζ�������Ϊ������ԭ�ζ��������ڱ���Һ�ı궨����Na2S2O3��I2ˮ��Һ�����Լ�ά����C�ĵζ������У��ζ��ķ�ʽ��������ġ�����źͲ�����DZ���Һ�ı궨�����в�������û��ζ��ķ�ʽ������
Cr2O72-����������3I2������������6S2O32-
0.01667mol/L ��10.00mL ������c(S2O32-)��19.76mL
��I2����Һ���õ���ֱ�ӵζ�����
I2 �� 2S2O32��
c(I2)��10.15mL c(S2O32-)��10.00mL
ͨ�����Ϲ�ϵ���ɷֱ�������ֱ���Һ��Ũ�ȣ�Na2S2O3����Һ��Ũ��Ϊ0.05062 mol/L��I2����Һ��Ũ��Ϊ0.02494 mol/L��
��ά����C�ĵζ������У����ݰ뷴Ӧ�е�ʧ������Ŀ����д������I2�Ĺ�ϵʽ��
I2 �� C6H8O6
c(I2)��V n(C6H8O6)
Ȼ�����ά����C���������������ͨ����ƽ��ֵ�ķ�������СżȻ��
Cr2O72-����������3I2������������6S2O32-
0.01667mol/L ��10.00mL ������c(S2O32-)��19.76mL
��I2����Һ���õ���ֱ�ӵζ�����
I2 �� 2S2O32��
c(I2)��10.15mL c(S2O32-)��10.00mL
ͨ�����Ϲ�ϵ���ɷֱ�������ֱ���Һ��Ũ�ȣ�Na2S2O3����Һ��Ũ��Ϊ0.05062 mol/L��I2����Һ��Ũ��Ϊ0.02494 mol/L��
��ά����C�ĵζ������У����ݰ뷴Ӧ�е�ʧ������Ŀ����д������I2�Ĺ�ϵʽ��
I2 �� C6H8O6
c(I2)��V n(C6H8O6)
Ȼ�����ά����C���������������ͨ����ƽ��ֵ�ķ�������СżȻ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
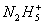 +4Fe3+
+4Fe3+ 4Fe2++Y+�������ݴ˿�֪YΪ( )
4Fe2++Y+�������ݴ˿�֪YΪ( ) 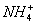
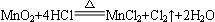 ��
��