��Ŀ����
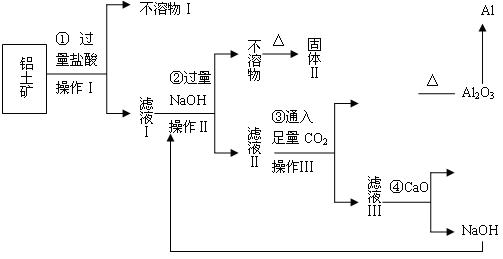
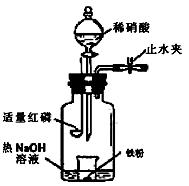
��12�֣�����������ij�������������ӻʼ���Ʒ����������ִ������캽�����졢������������ҵ����Ҫ���ϡ���ҵ����������(��Ҫ�ɷ�ΪAl2O3��Fe2O3��SiO2��)��ȡ��Al2O3��ұ������ԭ�ϣ�ij�о���ѧϰС�������������ȡ����ͼ
��1�������Ļ�ѧʽΪ_ �� ���ù������ɫΪ �� ��
��2����ʵ�ʹ�ҵ�������̢�����������ʯ��Ŀ���� �� ��
��3��д�����̢۵����ӷ���ʽ �� ��
��4����������������ĩ����ȼ�����³��������Ӹֹ죬��Ҫ�����ø÷�Ӧ �� ��
��5��������������ȡ���Ĺ��̲��漰�Ļ�ѧ��Ӧ������ �� ��
��6�����������г�NaOH��H2O����ѭ��ʹ���⣬������ѭ��ʹ�õ�������_ �� _��
��7��������Һ������ε���NaOH��Һ������������������NaOH�����ϵ��ȷ����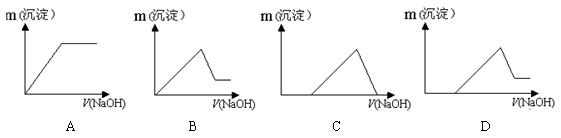
|
|

��1�������Ļ�ѧʽΪ_ �� ���ù������ɫΪ �� ��
��2����ʵ�ʹ�ҵ�������̢�����������ʯ��Ŀ���� �� ��
��3��д�����̢۵����ӷ���ʽ �� ��
��4����������������ĩ����ȼ�����³��������Ӹֹ죬��Ҫ�����ø÷�Ӧ �� ��
��5��������������ȡ���Ĺ��̲��漰�Ļ�ѧ��Ӧ������ �� ��
| A�����ֽⷴӦ | B��������ԭ��Ӧ | C���û���Ӧ | D���ֽⷴӦ |
��7��������Һ������ε���NaOH��Һ������������������NaOH�����ϵ��ȷ����

��12�֣���1��Fe2O3 ��1�֣� �� ����ɫ ��1�֣�
��2������Al2O3���۵㣬������������ ��1�֣�
��3��AlO2����2H2O �� CO2 = Al(OH)3��+HCO3�� ��2�֣�
��4���ų��������� ��1�֣�
��5�� C ��2�֣� ����6�� CaO CO2 ��2�֣�����7��D ��2�֣�
��2������Al2O3���۵㣬������������ ��1�֣�
��3��AlO2����2H2O �� CO2 = Al(OH)3��+HCO3�� ��2�֣�
��4���ų��������� ��1�֣�
��5�� C ��2�֣� ����6�� CaO CO2 ��2�֣�����7��D ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 ���������� ��
���������� ��


 �����ű�ʾ ����
�����ű�ʾ ���� _______________________________________________________________________
_______________________________________________________________________ ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽��



 �� �� ��
�� �� �� ����ʱ��Ӧ��Ũ���Ỻ��ע��ʢ��ˮ����Ͳ��
����ʱ��Ӧ��Ũ���Ỻ��ע��ʢ��ˮ����Ͳ��