��Ŀ����
12���㶹����һ����Ȼ���ϣ������ں��㶹��������ֲ���У���ҵ�ϳ���ˮ��ȩ���������ڴ��������¼��ȷ�Ӧ�Ƶã�
�������ɼױ�Ϊԭ�������㶹�ص�һ�ֺϳ�·�ߣ����ַ�Ӧ����������������ȥ��

A�������ֲ�ͬ��ѧ�������⣻
��ͬһ��̼ԭ�������������ǻ�ͨ�����ȶ�������ˮ�γ��ʻ���
��ش��������⣺
��1���㶹�صķ���ʽΪC9H6O2��
��2���ɼױ�����A�ķ�Ӧ����Ϊȡ����Ӧ��A�Ļ�ѧ����Ϊ2-�ȼױ��������ȼױ�����
��3����B����C�Ļ�ѧ��Ӧ����ʽΪ
 ��
����4��B��һ��ͬ���칹���к���1��������һ���ǻ�����ֻ��һ����������д�����Ľṹ��ʽ
 ���ں˴Ź��������г���5��壮
���ں˴Ź��������г���5��壮
���� �ױ��ڴ������·���������ȡ����Ӧ����A��A�������ֲ�ͬ��ѧ�������⣬����㶹�صĽṹ����֪AΪ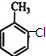 ���Ա�A��B����ʽ��֪��A��Clԭ�ӱ�-OHȡ������BΪ
���Ա�A��B����ʽ��֪��A��Clԭ�ӱ�-OHȡ������BΪ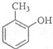 ���Ա�B��C�ķ���ʽ�����ˮ��ȩ���������ڴ����Ʊ��㶹�صķ�Ӧ����֪B�ڹ��������·���ȡ������CΪ
���Ա�B��C�ķ���ʽ�����ˮ��ȩ���������ڴ����Ʊ��㶹�صķ�Ӧ����֪B�ڹ��������·���ȡ������CΪ ��C����ˮ�ⷴӦ�õ�DΪ
��C����ˮ�ⷴӦ�õ�DΪ ���ݴ˽��
���ݴ˽��
��� �⣺�ױ��ڴ������·���������ȡ����Ӧ����A��A�������ֲ�ͬ��ѧ�������⣬����㶹�صĽṹ����֪AΪ ���Ա�A��B����ʽ��֪��A��Clԭ�ӱ�-OHȡ������BΪ
���Ա�A��B����ʽ��֪��A��Clԭ�ӱ�-OHȡ������BΪ ���Ա�B��C�ķ���ʽ�����ˮ��ȩ���������ڴ����Ʊ��㶹�صķ�Ӧ����֪B�ڹ��������·���ȡ������CΪ
���Ա�B��C�ķ���ʽ�����ˮ��ȩ���������ڴ����Ʊ��㶹�صķ�Ӧ����֪B�ڹ��������·���ȡ������CΪ ��C����ˮ�ⷴӦ�õ�DΪ
��C����ˮ�ⷴӦ�õ�DΪ ��
��
�⣺��1�����㶹�صĽṹ��ʽ��֪�������ʽΪC9H6O2��
�ʴ�Ϊ��C9H6O2��
��2���ױ�����������ȡ����Ӧ���� �����Կ�����һ����ԭ��ȡ��һ����ԭ�ӣ�����Ϊ2-�ȼױ��������ȼױ�����
�����Կ�����һ����ԭ��ȡ��һ����ԭ�ӣ�����Ϊ2-�ȼױ��������ȼױ�����
�ʴ�Ϊ��ȡ����Ӧ��2-�ȼױ��������ȼױ�����
��3����B����C�Ļ�ѧ��Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4��B�� ����һ��ͬ���칹���к���1��������һ���ǻ�����ֻ��һ�����������Ľṹ��ʽΪ
����һ��ͬ���칹���к���1��������һ���ǻ�����ֻ��һ�����������Ľṹ��ʽΪ ���ں˴Ź��������г���5��壬
���ں˴Ź��������г���5��壬
�ʴ�Ϊ�� ��5��
��5��
���� ���⿼���л���ĺϳ����ƶϣ�ע����ݷ�Ӧ�������л������ʽ�뷴Ӧ��Ϣ�����ƶϣ�������ѧ���ķ����������ƶ������Ŀ��飬�Ѷ��еȣ�

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�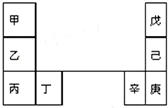 ��--����Ԫ�������ڱ��е����λ����ͼ�������ԭ���������3�����һ�ֵ�������Ȼ��Ӳ���������ʣ���������ͬ����Ԫ�أ������ж���ȷ���ǣ�������
��--����Ԫ�������ڱ��е����λ����ͼ�������ԭ���������3�����һ�ֵ�������Ȼ��Ӳ���������ʣ���������ͬ����Ԫ�أ������ж���ȷ���ǣ�������| A�� | �����ԣ��ף��ң��� | |
| B�� | ԭ�Ӱ뾶������������ | |
| C�� | �������ԭ�Ӻ�����������14 | |
| D�� | �ҵĵ����ڿ�����ȼ������ֻ�����Ӽ��Ļ����� |
| ѡ�� | ������ | �����Լ�����Һ�� | �����Լ�������Ӧ�����ӷ���ʽ |
| A | SO42-��Fe2+��NO3-��K+ | K3[Fe��CN��6] | 3Fe2++2[Fe��CN��6]3-=Fe3[Fe��CN��6]2�� |
| B | Na+��Fe3+��I-��ClO- | HCl | ClO-+H+=HClO |
| C | Ba2+��Al3+��Cl-��H+ | ����NaOH | Al3++3OH-=Al��OH��3�� |
| D | S2O32-��Na+��Cl-��SO32-��K+ | �������� | 2S2O32-+2H+=SO42-+3S��+H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | C��D��E��A��B | B�� | E��C��D��A��B | C�� | B��A��D��C��E | D�� | B��A��E��D��C |
| A�� | s��Ԫ�ض��ǽ���Ԫ�� | B�� | p��Ԫ��ȫ�����Ƿǽ���Ԫ�� | ||
| C�� | d����ds����f���ɸ���Ԫ����� | D�� | s����p���������0��Ԫ����� |
| A�� | �����Ӳ��Ե��� | B�� | ������������Ϊ4 | ||
| C�� | ����Ԫ�ص���������ͬ | D�� | ��������������ԭ�Ӵ� |
| A�� | ����Ԫ����ǽ���Ԫ�ص�ԭ�Ӽ�ֻ�γ����Ӽ� | |
| B�� | ԭ�ӻ����Ӽ�����������л�ѧ�� | |
| C�� | �ǽ���Ԫ�ؼ�ֻ���γɹ��ۼ� | |
| D�� | ���ۻ������ڲ������м��Լ��ͷǼ��Լ� |
| A�� | ǿ������Һ�У�NH4+��K+��CO32-��Cl- | |
| B�� | ��SO42-���ڵ���Һ�У�Na+��Mg2+��Ba2+��I- | |
| C�� | ���������ܷų���������Һ�У�K+��Ba2+��Cl-��Br- | |
| D�� | ��ǿ���Ե���Һ�У�NH4+��Na+��Cl-��H+ |