ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ(1)“―÷Σ≥ΘΈ¬≥Θ―Ιœ¬ΘΚ
ΔΌCH3OH(l)+O2(g)®TCO(g)+2H2O(g) ΓςH=Θ≠359.8kJΓΛmolΘ≠1
ΔΎ2CO(g)+O2(g)=2CO2(g) ΓςH=Θ≠556.0kJΓΛmolΘ≠1
ΔέH2O(g)=H2O(l) ΓςH=Θ≠44.0kJΓΛmolΘ≠1
–¥≥ωΧεœ÷ΦΉ¥Φ»Φ…’»»ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ________________________________ΓΘ
(2)Ρ≥Έ¬Ε» ±Θ§ΫΪ2 mol CO”κ5 mol H2ΒΡΜλΚœΤχΧε≥δ»κ»ίΜΐΈΣ2 LΒΡΟή±’»ίΤς÷–Θ§‘Ύ¥ΏΜ·ΦΝΒΡΉς”Οœ¬ΖΔ…ζΖ¥”ΠΘΚCO(g)ΘΪ2H2(g) ![]() CH3OH(g)ΓΘ
CH3OH(g)ΓΘ
ΔΌΨ≠Ιΐ5 minΚσΘ§Ζ¥”Π¥οΒΫΤΫΚβΘ§¥Υ ±ΉΣ“ΤΒγΉ”6 molΓΘΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣ________ΓΘv(H2)ΘΫ________ mol/(LΓΛmin)ΓΘ»τ±Θ≥÷ΧεΜΐ≤Μ±δΘ§‘Ό≥δ»κ2 mol COΚΆ1.5 mol CH3OHΘ§¥Υ ±v(’ΐ)________v(Ρφ)(ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑΘΫΓ±)ΓΘ
ΔΎ‘ΎΤδΥϊΧθΦΰ≤Μ±δΒΡ«ιΩωœ¬Θ§‘Ό‘ωΦ”2 mol CO”κ5 mol H2Θ§¥οΒΫ–¬ΤΫΚβ ±Θ§COΒΡΉΣΜ·¬ ________(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
Δέœ¬Ν–≤ΜΡήΥΒΟςΗΟΖ¥”Π“―¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «________ΓΘ
a.CH3OHΒΡ÷ ΝΩ≤Μ±δ b.ΜλΚœΤχΧεΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ≤Μ‘ΌΗΡ±δ c.vΡφ(CO)ΘΫ2v’ΐ(H2) d.ΜλΚœΤχΧεΒΡΟήΕ»≤Μ‘ΌΖΔ…ζΗΡ±δ
ΓΨ¥πΑΗΓΩCH3OH(l)+3/2O2(g)®TCO2(g)+2H2O(l) ΓςH=Θ≠725.8kJΓΛmolΘ≠1 3 0.3 > ‘ω¥σ cd
ΓΨΫβΈωΓΩ
ΗυΨί»Φ…’»»ΒΡΗ≈ΡνΓΔάϊ”ΟΗ«ΥΙΕ®¬… ι–¥»»Μ·―ßΖΫ≥Χ ΫΘΜΗυΨίΜ·―ßΤΫΚβ≥Θ ΐΓΔΜ·―ßΖ¥”ΠΥΌ¬ ΓΔΉΣΜ·¬ ΒΡΕ®“εΫχ––ΦΤΥψΘΜΗυΨίΜ·―ßΤΫΚβΒΡ±Ψ÷ ΚΆΧΊ’ς≈–ΕœΤΫΚβ±ξ÷ΨΘΜΗυΨί≈®Ε»…Χ”κKΒΡΙΊœΒ≈–ΕœΤΫΚβ“ΤΕ·ΖΫœρΓΘ
(1)ΗυΨί»Φ…’»»Η≈ΡνΘ§Χεœ÷ΦΉ¥Φ»Φ…’»»ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ÷–CH3OH(l)”ΠΈΣ1molΘ§«“…ζ≥…CO2(g)ΓΔH2O(l)ΓΘάϊ”ΟΗ«ΥΙΕ®¬…Θ§ΫΪΔΌ+ΔΎΓΝ+ΔέΓΝ2ΒΟCH3OH(l)+3/2O2(g)®TCO2(g)+2H2O(l) ΓςH=Θ≠725.8kJΓΛmolΘ≠1ΓΘ
(2)ΉΣ“ΤΒγΉ”6 molΘ§‘ρΖ¥”ΠœϊΚΡ1.5mol CO(g)ΓΔ3mol H2(g)Θ§…ζ≥…1.5mol CH3OH(g)ΓΘ
Ρ≥Έ¬Ε»ΓΔ2 L CO(g)ΘΪ2H2(g) ![]() CH3OH(g)
CH3OH(g)
Τπ Φ/molΘΚ 2 5 0
ΉΣΜ·/molΘΚ 1.5 3 1.5
5minΤΫΚβ/molΘΚ 0.5 2 1.5
ΔΌΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKΘΫ![]() ΘΫ3Θ§v(H2)ΘΫ
ΘΫ3Θ§v(H2)ΘΫ![]() ΘΫ0.3mol/(LΓΛmin)ΓΘ
ΘΫ0.3mol/(LΓΛmin)ΓΘ
±Θ≥÷ΧεΜΐ≤Μ±δΘ§‘Ό≥δ»κ2 mol COΚΆ1.5 mol CH3OH ±Θ§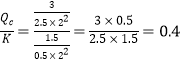 Θ§Ι v(’ΐ)>v(Ρφ)ΓΘ
Θ§Ι v(’ΐ)>v(Ρφ)ΓΘ
ΔΎΤδΥϊΧθΦΰ≤Μ±δ ±Θ§‘Ό‘ωΦ”2 mol CO”κ5 mol H2ΥυΫ®ΝΔΒΡ–¬ΤΫΚβΘ§œύΒ±”ΎΝΫΗω‘≠ΤΫΚβΉ¥Χ§‘ΎΧεΜΐΦθΑκΘ®Φ”―ΙΘ© ± ΙΤΫΚβ”““ΤΘ§Ι COΉΣΜ·¬ ‘ω¥σΓΘ
Δέa.CH3OH÷ ΝΩ≤Μ±δ ±Θ§ΤδΥϋΗςΈο÷ ΒΡ÷ ΝΩ“≤≤Μ±δΘ§ΈΣΜ·―ßΤΫΚβΉ¥Χ§ΘΜb.ΜλΚœΤχΧεΒΡΉή÷ ΝΩ≤Μ±δΘ§ΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ≤Μ‘ΌΗΡ±δ±μΟςΤχΧεΉήΈο÷ ΒΡΝΩ≤Μ±δΘ§‘ρΈΣΜ·―ßΤΫΚβΉ¥Χ§ΘΜc.ΫΪvΡφ(CO):vΡφ(H2)ΘΫ1:2¥ζ»κvΡφ(CO)ΘΫ2v’ΐ(H2)Θ§ΒΟvΡφ(H2)ΘΫ4v’ΐ(H2)Θ§Ι ≤Μ «Μ·―ßΤΫΚβΘΜd.ΜλΚœΤχΧεΒΡΉή÷ ΝΩΓΔ»ίΤςΧεΜΐΕΦ≤Μ±δΘ§‘ρΟήΕ»±Ί»Μ≤Μ±δΘ§≤ΜΡήΉςΤΫΚβ±ξ÷ΨΘ§―ΓcdΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ