��Ŀ����
����Ŀ��K2FeO4��һ�ּ�������������������������ɱ���������ȹ�����һ�����ɫ��Чˮ���������ѳ�Ϊ������ɶ�ȱ������֮һ����ҵ�Ʊ�������ͼ��ʾ���ش��������⣺

��1����ʪ���Ʊ�Na2FeO4�����У���д���ȼҵ�������ö��Ե缫��ⱥ��NaCl��Һ�����ӷ���ʽ��___��NaClO��FeCl3�ڼ����������Ʊ�Na2FeO4�ķ�Ӧ�б�������ԭ���뱻��ԭ��ԭ�Ӹ�����Ϊ____����ѭ��ʹ�õ�����Ϊ___����дһ�֣���
��2����ⷨ�Ʊ�Na2FeO4�����У���������Ϊ��ƽ�壬��ʹ��ǰҪ������ɰ��ϸɰ��ĥ�⣬��Ŀ��Ϊ____���ѺϽ�������������������ҺΪNaOH��Һ����д�������缫��Ӧʽ��____��
��3���ɷ��Ʊ�Na2FeO4���������緢�ֵ��Ʊ����գ���д��NaClO���塢Fe(NO3)3��9H2O���塢NaOH�����ڸ������ڵ��������Ʊ�Na2FeO4�Ļ�ѧ����ʽ��_____��
��4����д����Na2FeO4�Ʊ�K2FeO4�Ļ�ѧ����ʽ��____����˵���÷�Ӧ�ܷ�����ԭ��____��
��5����K2FeO4�ᴿʱ��-5�汥��KOH��ԭ��____��K2FeO4����ɫˮ���������ܶ�ˮ��ɱ����������������ˮ�����ã���ԭ��Ϊ_____��
���𰸡�2Cl+2H2O![]() 2OH+Cl2��+H2�� 2��3 NaCl��NaOH��KOH ��ȥ�缫������������ƽ�壩��������������Ĥ Fe-6e+8OH��FeO42-+4H2O 2Fe(NO3)3��9H2O+10NaOH+3NaClO
2OH+Cl2��+H2�� 2��3 NaCl��NaOH��KOH ��ȥ�缫������������ƽ�壩��������������Ĥ Fe-6e+8OH��FeO42-+4H2O 2Fe(NO3)3��9H2O+10NaOH+3NaClO![]() 2Na2FeO4+3NaCl+23H2O+6NaNO3 Na2FeO4+2KOH��K2FeO4��+2NaOH K2FeO4�ڼ�����Һ�е��ܽ�ȱ�Na2FeO4С�ö࣬�ʿɳ������� ����K2FeO4�ܽ�ȣ����ٲ�Ʒ����� K2FeO4��ǿ�������ԣ���ˮ����ɱ�������������ã�������Ӧ�����ɵ�Fe3+����ˮ���γ�Fe(OH)3���壬���������ã����ܴﵽ��ˮ��Ŀ��
2Na2FeO4+3NaCl+23H2O+6NaNO3 Na2FeO4+2KOH��K2FeO4��+2NaOH K2FeO4�ڼ�����Һ�е��ܽ�ȱ�Na2FeO4С�ö࣬�ʿɳ������� ����K2FeO4�ܽ�ȣ����ٲ�Ʒ����� K2FeO4��ǿ�������ԣ���ˮ����ɱ�������������ã�������Ӧ�����ɵ�Fe3+����ˮ���γ�Fe(OH)3���壬���������ã����ܴﵽ��ˮ��Ŀ��
��������
(1)�ȼҵ�е�ⱥ��ʳ��ˮ���ڷ��������ͣ����ӷ���ʽΪ2Cl+2H2O![]() 2OH+Cl2��+H2����NaClO��FeCl3�Ʊ�Na2FeO4��Ӧ����NaClO��FeCl3������Na2FeO4���Ƚ���2�ۣ�������3�ۣ���������ԭ���뱻��ԭ��ԭ�Ӹ�����Ϊ2��3�����ɴ�K2FeO4ʱ������NaOH��������ѭ���Ʊ�NaClO���Ʊ�NaClOʱ��NaCl���ɣ�NaCl������ѭ���Ʊ�NaOH��K2FeO4�ᴿ���KOH��������ȡ��K2FeO4��
2OH+Cl2��+H2����NaClO��FeCl3�Ʊ�Na2FeO4��Ӧ����NaClO��FeCl3������Na2FeO4���Ƚ���2�ۣ�������3�ۣ���������ԭ���뱻��ԭ��ԭ�Ӹ�����Ϊ2��3�����ɴ�K2FeO4ʱ������NaOH��������ѭ���Ʊ�NaClO���Ʊ�NaClOʱ��NaCl���ɣ�NaCl������ѭ���Ʊ�NaOH��K2FeO4�ᴿ���KOH��������ȡ��K2FeO4��
(2)��ƽ���ڷ��ù����У�����������ۣ�����������ʹ��ǰҪ������ɰ��ϸɰ��ĥ���dz�ȥ��������������Ĥ���������ҺΪNaOH��Һ����������������Na2FeO4��������ӦʽΪ��Fe-6e+8OH��FeO42-+4H2O��
(3)��Ӧ����ﶼ�Ѹ���������Fe��+3�����ߵ�+6�ۣ�Cl��+1�۽���-1�ۣ����û��ϼ������غ���ƽ����ʽ��2Fe(NO3)3��9H2O+10NaOH+3NaClO![]() 2Na2FeO4+3NaCl+23H2O+6NaNO3��
2Na2FeO4+3NaCl+23H2O+6NaNO3��
(4)�ӹ������̿��Կ�������KOH��Na2FeO4ת���K2FeO4�����Է�ӦΪNa2FeO4+2KOH��K2FeO4��+2NaOH��K2FeO4�ڼ�����Һ�е��ܽ�ȱ�Na2FeO4С�ö࣬�ʿɳ���������
(5)�¶�Խ�ͣ�K2FeO4�ܽ��ԽС��K2FeO4��ǿ�������ԣ���ʹ�����ʱ��ԣ���ˮ��ɱ�������������ã�������Ӧ�����ɵ�Fe3+�ܷ���ˮ���γ�Fe(OH)3���壬���������ã����ܴﵽ��ˮ��Ŀ�ġ�

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�����Ŀ��ij�����İ�����ˮ����Ҫ����![]() �����õ绯ѧ���������Դ�����
�����õ绯ѧ���������Դ�����
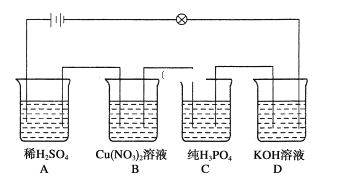
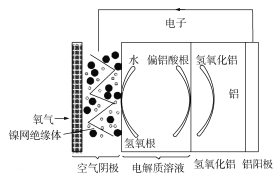
��1��ͼ1�ǵ绯ѧ��������ԭ��ʾ��ͼ��a�ĵ缫��Ӧʽ��_____________��
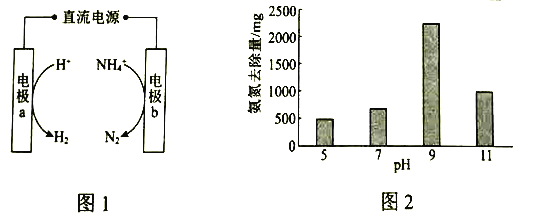
��2���о���ʾ��������������ʱ����ͬ![]() �°�����ȥ������ͼ2��ʾ����֪��
�°�����ȥ������ͼ2��ʾ����֪��![]() ��
��![]() ��ȣ��ڵ缫���������Ч�����á����ƽ���ƶ�ԭ����ͼ�����ݣ�����
��ȣ��ڵ缫���������Ч�����á����ƽ���ƶ�ԭ����ͼ�����ݣ�����![]() ��5��9ʱ����ȥ���������ԭ��______________��
��5��9ʱ����ȥ���������ԭ��______________��
��3���ڵ���ˮ�Ĺ����У�![]() �ᾭ����
�ᾭ����![]() ���Ĺ��̡�����������ͬ��
���Ĺ��̡�����������ͬ��![]() ��Ũ�Ȳ�ͬʱ����ˮ�а����ѳ�Ч�ʵ�ʵ�������£�
��Ũ�Ȳ�ͬʱ����ˮ�а����ѳ�Ч�ʵ�ʵ�������£�
| 400 | 100 |
���ʱ��/h | 0.5 | 0.5 |
�����ѳ�Ч��/�� | 2.40.8 |
������������ͬ���ʵ����![]() ��Ũ�ȣ��������������ˮ��
��Ũ�ȣ��������������ˮ��![]() ���ѳ�Ч�ʡ�
���ѳ�Ч�ʡ�
�û�ѧ�������ԭ��____________��![]() ��
��
��ͼ2�У�![]() ʱ����ˮ��
ʱ����ˮ��![]() ȥ�����½������ܵ�ԭ���ǣ�_______________��
ȥ�����½������ܵ�ԭ���ǣ�_______________��