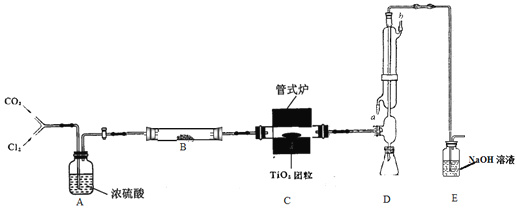
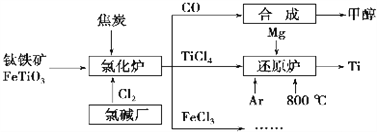
��Ŀ����
����Ŀ��84����Һ(��Ч�ɷ��Ǵ�������)��Ư��(��Ч�ɷ��Ǵ������)���������ճ������г��õ����������㷺Ӧ�����ճ������С���������Ҫ��ش�������⣺
(1)��0.1 mol��L��1��84����Һ�еμӼ��η�̪��Һ�����ܹ۲쵽��������______________��д����Ӧ�����ӷ���ʽ��______________________________��
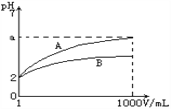
(2)ijͬѧ�ⶨƯ����ҺpH�IJ�����������һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������ֽ�ϣ���Լ����Ӻ��������ɫ�����ա�����Ϊ���ܷ�ﵽʵ��Ŀ�ģ�________(��ܡ���)��
(3)���й���0.5 mol��L��1 NaClO��Һ�и�����Ũ�ȵĹ�ϵʽ��ȷ����____________��
A��c(OH��)>c(H��)>c(Na��)>c(ClO��) B��c(Na��)��c(ClO��)��c(HClO)
C��c(Na��)>c(ClO��)>c(OH��)>c(H��) D��c(Na��)��c(H��)��c(ClO��)
���𰸡� ��Һ��ɫ�ȱ�����ɫ ClO����H2O![]() HClO��OH�� �� BC
HClO��OH�� �� BC
����������1������������Һ����ǿ�����ԣ������������ˮ����Һ�Լ��ԣ�����0.1 mol��L��1��84����Һ�еμӼ��η�̪��Һ�����ܹ۲쵽����������Һ��ɫ�ȱ�����ɫ����Ӧ�����ӷ���ʽΪClO����H2O![]() HClO��OH������2�����ڴ��������Һ����ǿ�����ԣ���Ư�����ָʾ������˲�����pH��ֽ����pH����3��A�������������ˮ����Һ�Լ��ԣ���c(Na��)>c(ClO��)>c(OH��)>c(H��)��A����B�����������غ��֪c(Na��)��c(ClO��)��c(HClO)��B��ȷ��C�������������ˮ����Һ�Լ��ԣ���c(Na��)>c(ClO��)>c(OH��)>c(H��)��C��ȷ��D�����ݵ���غ��֪c(Na��)��c(H��)��c(ClO��)��c(OH��)��D����ѡBC��
HClO��OH������2�����ڴ��������Һ����ǿ�����ԣ���Ư�����ָʾ������˲�����pH��ֽ����pH����3��A�������������ˮ����Һ�Լ��ԣ���c(Na��)>c(ClO��)>c(OH��)>c(H��)��A����B�����������غ��֪c(Na��)��c(ClO��)��c(HClO)��B��ȷ��C�������������ˮ����Һ�Լ��ԣ���c(Na��)>c(ClO��)>c(OH��)>c(H��)��C��ȷ��D�����ݵ���غ��֪c(Na��)��c(H��)��c(ClO��)��c(OH��)��D����ѡBC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�