��Ŀ����
11��ijͬѧ��ͼ��ʾװ����ȡ�屽�������飮��֪����CH3CH2OH+HBr-CH3CH2Br+H2O��������Ϊ��ɫҺ�壬������ˮ���е�Ϊ38.4�棬�۵�Ϊ-119�森
��Ҫʵ�鲽�����£�
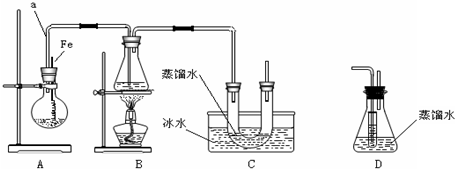
����װ��װ�ã�����������ԣ�
������ƿ�м���һ��������Һ�壬����ƿ�м����Ҵ����Ը��ڽ������ܿڴ�����U���м�������ˮ��ס�ܵף���ˮ���м����ˮ��
�۽�Aװ���еĴ���˿С�����²��뱽��Һ��Ļ��Һ�У�
�ܵ�ȼBװ���еľƾ��ƣ���С������ƿ����10���ӣ�
����д���пհף�
��1����д��Aװ�������������л���Ӧ��ѧ����ʽ
A��
 ��
����2��Cװ����U���ڲ�������ˮ��ס�ܵ��������ܽ������廯�����壬��ֹ�廯�⼰�����ݳ���Ⱦ������
��3����Ӧ��Ϻ�U����Һ��ֲ㣬���������£���ϻ��£��㣮
��4��Ϊ֤����ͱ���������Ӧ��ȡ����Ӧ�����Ǽӳɷ�Ӧ����ѧ����װ��D����װ��B��Cֱ����A�������²���ʵ�飮
��װ��D����ƿ�У�С�Թ��ڵ�Һ���DZ��������Ȼ�̼��������һ�ַ��������Լ������ƣ�������Ҫ���������ջӷ���������������
�ڷ�Ӧ������ƿ�еμ���������Һ������ɫʯ����Һ��������һ�ַ��������Լ������ƣ������е���ɫ��������������ɫʯ����Һ��죩֤���÷�ӦΪȡ����Ӧ��
��5��Ҫ����ij�������е���Ԫ�أ���ȷ��ʵ�鷽����D
A���������Ƶ���ˮ���ټ�������CCl4���۲��²��Ƿ��Ϊ�Ⱥ�ɫ
B��������������Һ���ټ���ϡ����ʹ��Һ�����ԣ��۲�����dz��ɫ��������
C������NaOH��Һ���ȣ���ȴ�������������Һ���۲�����dz��ɫ��������
D������NaOH��Һ���ȣ���ȴ�����ϡ����ʹ��Һ�����ԣ��ٵ�����������Һ���۲�����dz��ɫ�������ɣ�
���� ��1��Aװ���б���Һ�������۴������·���ȡ����Ӧ�����屽���廯�⣻
��2����������ǿ�ᣬˮ������ֹ���ʵ��������ˮ������ã�
��3����������ܶȴ���ˮ��
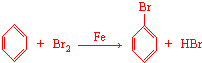
��4����ͼ��֪��A�з��� +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$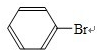 +HBr��װ��D����ƿ��С�Թ��ڱ��������Ȼ�̼�������壬��ƿ�ڵ�Һ��Ϊˮ������HBr���壬Ȼ��μ����������������ӣ�
+HBr��װ��D����ƿ��С�Թ��ڱ��������Ȼ�̼�������壬��ƿ�ڵ�Һ��Ϊˮ������HBr���壬Ȼ��μ����������������ӣ�
��5������ij�������е���Ԫ�أ����Խ����������ˮ�⣬�ٽ���Һ�������ԣ��ټ��������Ӽ��ɣ�
��� �⣺��1��Aװ���У��ڴ����������£������ϵ���ԭ�ӱ���ԭ����ȡ���������屽��ͬʱ���廯�����ɣ� ��
��
�ʴ�Ϊ�� ��
��
��2���廯�⼫������ˮ���γɵ���������ǿ�ᣬˮ������ֹ�ӷ�����HBr���������ˮ������ã�
�ʴ�Ϊ���ܽ������廯�����壬��ֹ�廯�⼰�����ݳ���Ⱦ������
��3����������ܶȴ���ˮ�����Է�Ӧ��Ϻ�U����Һ��ֲ㣬���������²㣬
�ʴ�Ϊ���£�
��4����ͼ��֪��A�з��� +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$ +HBr��װ��D����ƿ��С�Թ��ڱ��������Ȼ�̼�������壬��ƿ�ڵ�Һ��Ϊˮ������HBr���壬Ȼ��μ����������������ӣ����Ԣ�װ��D����ƿ�У�С�Թ��ڵ�Һ���� CCl4������Ҫ���������ջӷ����������������ڷ�Ӧ������ƿ�еμ� ��������Һ������ɫʯ����Һ��������������Һ������ɫʯ����Һ��֤���÷�ӦΪȡ����Ӧ��
+HBr��װ��D����ƿ��С�Թ��ڱ��������Ȼ�̼�������壬��ƿ�ڵ�Һ��Ϊˮ������HBr���壬Ȼ��μ����������������ӣ����Ԣ�װ��D����ƿ�У�С�Թ��ڵ�Һ���� CCl4������Ҫ���������ջӷ����������������ڷ�Ӧ������ƿ�еμ� ��������Һ������ɫʯ����Һ��������������Һ������ɫʯ����Һ��֤���÷�ӦΪȡ����Ӧ��
�ʴ�Ϊ�����������Ȼ�̼�������ջӷ�����������������������Һ������ɫʯ����Һ�����е���ɫ��������������ɫʯ����Һ��죩��
��5������ij�������е���Ԫ�أ����Խ����������ˮ�⣬�ٽ���Һ�������ԣ��ټ��������Ӽ��ɣ������Ϊ����NaOH��Һ���ȣ���ȴ�����ϡ����ʹ��Һ�����ԣ��ٵ�����������Һ���۲�����dz��ɫ�������ɣ�
��ѡD��
���� ���⿼�����屽�����������ȡ������һ���ۺ��Խ�ǿ��ʵ���⣬�����Ʊ����ֲ�Ʒ�屽�������飬��Ҫ�������⼰װ��ͼʾ����ϵ��ѧ֪ʶ����������ɣ������Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Ũ������ǿ����� | |
| B�� | ���������ˮ��Һ��һ��������ȫ���� | |
| C�� | ǿ����ʵ�ˮ��Һ�в����ڷ��� | |
| D�� | ���������Һ�ĵ�������һ������ |
����������������������ʽ��ȫ����ʱ��pH���£�
| ������ | ��ʼ���� | ��ȫ���� |
| Al��OH��3 | 3.8 | 5.2 |
| Fe��OH��3 | 2.7 | 3.2 |
| Fe��OH��2 | 7.6 | 9.7 |
| Ni��OH��2 | 7.1 | 9.2 |
��1������a��c����ʹ�õ�����������̨������Ȧ�����ƾ��ơ�©�������������Ҫ����Ҫ����Ϊ������
��2��������������з��������ӷ���ʽ��2Al+2OH-+2H2O�T2AlO2-+3H2����Al2O3+2OH-�T2AlO2-+H2O��
��3���������ʱ�����������H2SO4���ѧʽ�������������a���������ٺ���Һ�п��ܺ��еĽ���������Ni2+��Fe2+��
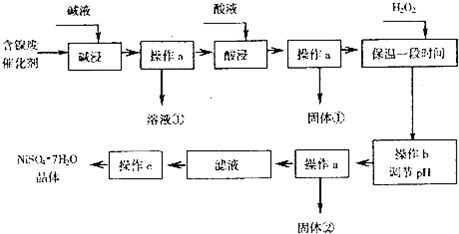
��4����40�����ң���6%��H2O2������Fe2+���⣬������NaClO3�����������ڽ�С��pH������ˮ�⣬��������һ��dz��ɫ�Ļ�������[Na2Fe6��SO4��4��OH��12]������������ȥ��ͼ���¶�-pHֵ�����ɵij�����ϵͼ��ͼ����Ӱ�����ǻ��������ȶ����ڵ�������֪25��ʱ��Fe��OH��3��Ksp=2.64��10-39��������˵����ȷ����CD��ѡ����ţ���
A��FeOOH����Ϊ+2��
B������25��ʱ����H2O2����Fe2+������pH=4ʱ��ȥ������ʱ��Һ��c��Fe3+��=2.64��10-29
C��������������������������Fe2+���ӷ���ʽΪ��6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O
D����ҵ�������¶ȳ�������85��95�����ɻ������ƣ���ʱˮ���pHԼΪ1.2��1.8
��5������b����pH�ķ�ΧΪ3.2-7.1��
��6����Ʒ��������ʱ����������̷���FeSO4•7H2O������ԭ�������H2O2���������㣨��H2O2ʧЧ��������ʱ�䲻�㵼��Fe2+δ����ȫ������ɵģ�
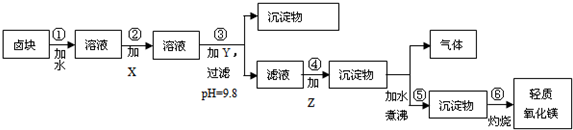
��Ҫ���Ʒ�����������ʣ����������ɱ��ϵͣ�����ݱ�1�ͱ�2�ṩ�����ϣ���д�հף�
��1 �����������������pH
| ���� | ��ʼ���� | ������ȫ |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6* |
| Mn��OH��2 | 8.3 | 9.8 |
| Mg��OH��2 | 9.6 | 11.1 |
��2 ԭ�ϼ۸��
| ���� | �۸�Ԫ•��-1�� |
| ƯҺ����25.2% NaClO�� | 450 |
| ˫��ˮ����30% H2O2�� | 2400 |
| �ռ��98% NaOH�� | 2100 |
| �����99.5% Na2CO3�� | 600 |
��2���ڲ�����м�����Լ����ռ֮����Ҫ����pH=9.8����Ŀ����ʹMg2+�������������ת���ɳ�����ȥ��
��3��ijȼ�ϵ�ص�ȼ��ΪCO��������Ϊ��CO2��O2�������Ϊ����̬���Լ�Z��������м����Լ�����Ч�ɷ֣������ȼ�ϵ�ص������缫��Ӧ����ʽΪ��O2+4e-+2CO2�T2CO32-��
��4���ڲ�����з����ķ�Ӧ��MgCO3+H2O $\frac{\underline{\;\;��\;\;}}{\;}$ Mg��OH��2+CO2����

��1���ٵ绡¯�з�������Ҫ��Ӧ��SiO2+2C$\frac{\underline{\;����\;}}{\;}$Si���ֹ裩+2CO����
����ʯӢɰ�ͽ�̿�ڵ绡¯�и��¼���Ҳ��������̼���裬�÷�Ӧ�Ļ�ѧ����ʽ
ΪSiO2+3C$\frac{\underline{\;����\;}}{\;}$SiC+2CO����̼�����ֳƽ��ɰ���侧��ṹ����ʯ���ƣ�
��2������������Ӧ�IJ����У�SiCl4��Լռ85%������Cl2�ȣ��й����ʵķе��������±���
| ���� | Si | SiCl4 | Cl2 |
| �е�/�� | 2355 | 57.6 | -34.1 |
A������-34.1��B������57.6��C������57.6��D��-34.1��
��3���ٷ�ĩ״Si3N4��ˮ������һ���д̼�����ζ��������������������һ�������Ե��ᣬ�÷�Ӧ�ķ���ʽ��Si3N4+9H2O=4NH3��+3H2SiO3����
�ڸù����������漰����Ҫ��Ӧ����������ԭ��Ӧ����2����
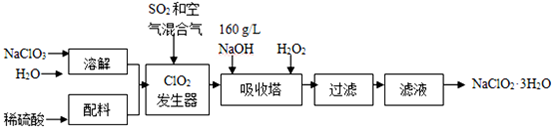
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2•3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10%���°�ȫ��
��160g•L-1 NaOH��Һ��ָ160g NaOH��������ˮ������Һ�����Ϊ1L��
��1��160g•L-1 NaOH��Һ�����ʵ���Ũ��Ϊ4mol/L����Ҫ�������Һ����������������Ҫ��һ����������Һ���ܶȣ�������˵������
��2���������й�����������ÿ�����b��ѡ����ţ���
a����SO2������SO3����ǿ���� b��ϡ��ClO2�Է�ֹ��ը c����NaClO3������ClO2
��3���������ڵķ�Ӧ�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2�����������¶Ȳ��ܳ���20�棬��Ŀ���Ƿ�ֹH2O2�ֽ⣮
��4������Һ�еõ�NaClO2•3H2O�־����ʵ�����������Ũ������ȴ�ᾧ������
Ҫ�õ�������NaClO2•3H2O���������еIJ������ؽᾧ����������ƣ�
��5������������֪������pH��2.0ʱ��ClO2-�ܱ�I-��ȫ��ԭ��Cl-��
��Һ��Na2S2O3����I2��Ӧ����NaI��Na2S4O6��
���ⶨ��Ʒ��NaClO2•3H2O�ĺ������ֽ������²�����
| ����I | ��ȡ��Ʒw g�����Һ������ƿ�У�������pH��2.0 |
| ����II | ����ƿ�м�������KI ���壬��ֽ��裬����������ָʾ�� |
| ����III | ��c mol/L��Na2S2O3��Һ�ζ� |
������дﵽ�ζ��յ�ʱ�������ǵ������һ�α���Һ����Һ����ɫ�仯Ϊ��ɫ�Ұ���Ӳ��仯��
���������ζ���������ȥ��V mL Na2S2O3��Һ������Ʒ��NaClO2•3H2O����������Ϊ$\frac{9.05��1{0}^{-2}Vc}{4W}$��100%������ĸ��ʾ����
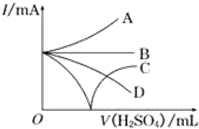 ��Ba��OH��2��Һ����μ���ϡ���ᣮ������������⣺
��Ba��OH��2��Һ����μ���ϡ���ᣮ������������⣺