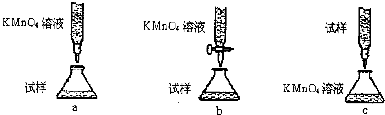
��Ŀ����
13����ţ���к���������ṹΪ
��1�����������������Ʒ�Ӧ�Ļ�ѧ����ʽ
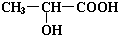 +2Na��
+2Na�� +H2��
+H2����2������������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ
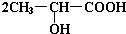 +Na2CO3��
+Na2CO3��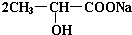 +H2O+CO2����
+H2O+CO2������3��3����������Ũ������������Ӧ��������Ϊ��״ʱ����ṹ��ʽΪ

��4��������������Ũ������������Ӧ��������Ϊ��״ʱ����ṹ��ʽΪ
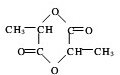
��5����������Ũ������������Ӧ�γ���״�߷��ӻ������ṹ��ʽΪ
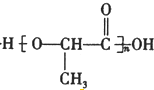
��6����������һ�������·�����ȥ��Ӧ�������л���Ľṹ��ʽΪ
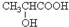 $��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
���� ��1������������ܺͽ����Ʒ����û���Ӧ����������
��2�������ܺ�̼���Ʒ�Ӧ�����κ�ˮ��
��3����������к����ǻ����Ȼ����ɷ���������Ӧ��
��4���������������Ӧ����Ϊ�����������ӷ���������Ӧ��
��5������ͨ��������Ӧ���е����۷�Ӧ���ɾ����
��6����������к��е��ǻ������ڵ�̼�ϵ��ⷢ����ȥ��Ӧ����ϩ����
��� �⣺��1�������еĴ��ǻ����Ȼ���������Ʒ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ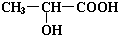 +2Na��
+2Na�� +H2����
+H2����
�ʴ�Ϊ��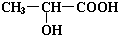 +2Na��
+2Na�� +H2����
+H2����
��2�������е��Ȼ��������ͨ�ԣ�������Na2CO3��Һ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ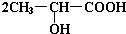 +Na2CO3��
+Na2CO3��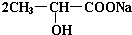 +H2O+CO2����
+H2O+CO2����
�ʴ�Ϊ��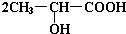 +Na2CO3��
+Na2CO3��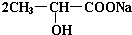 +H2O+CO2����
+H2O+CO2����
��3�������к����ǻ����Ȼ����ɷ���������Ӧ��3�������ᷢ��������Ӧ������ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4������Ľṹ��ʽΪCH3CH��OH��COOH��2�����������һ�������¿ɷ���������Ӧ������Ԫ��״�������Ӧ����ʽΪ��2CH3CH��OH��COOH$\stackrel{����}{��}$ +2H2O��
+2H2O��
�ʴ�Ϊ�� ��
��
��5������ͨ��������Ӧ���е����۷�Ӧ���ɾ����ᣬ��Ӧ����ʽΪ��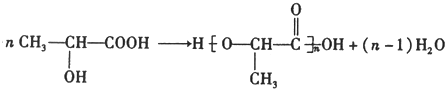 ��
��
��״�߷��ӻ�����Ϊ�����ᣬ��ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��������һ�������¿ɷ�����ȥ��Ӧ������CH2=CHCOOH����Ӧ�ķ���ʽΪ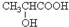 $��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
�ʴ�Ϊ��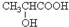 $��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
���� ����������ĽṹΪ���忼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע������л���Ľṹ�ص�����ŵ����ʣ���Ŀ�Ѷ��еȣ�

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�| ��Һ | ���� | ���� | |
| A | ���з�̪��������Һ | ���� | ��Һ��ɫ���� |
| B | ���з�̪�İ�ˮ | ��������NH4Cl���� | ��Һ��ɫ��dz |
| C | ���з�̪��CH3COONa��Һ | ��������NaOH���� | ��Һ��ɫ��dz |
| D | �Ȼ�����Һ | ���� | ��Һ��ɫ��dz |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ֯�� | �� | �� | �� |
| ����ʱ����ζ | �ս���ë����ζ | ��ֽ����ζ | �������ζ |
| A�� | H��D��16O��18O��Ϊͬλ�أ�H216O��D216O��H218O��D218O��Ϊͬ���칹�� | |
| B�� | ���ʯ��ʯī��Ϊ̼��ͬ�������壬�仯ѧ�������������ʾ�����ͬ | |
| C�� | 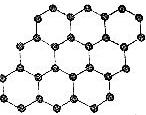 ʯīϩ���ṹ��ͼ��ʾ������̼ԭ�ӹ��ɵĵ���Ƭ״�ṹ���²��ϣ���ʯī��Ϊͬλ�� | |
| D�� | ������ԭ�ӿ����γ�H-����NaH�����������˽���ɽ���Ԫ������Ԫ�����ڱ��Ģ�A�� |