��Ŀ����
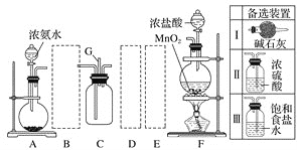
����Ŀ����84������Һ�ijɷ���NaClO��ij��ѧ�о���ѧϰС����ʵ�����Ʊ�NaClO��̽�������ʡ���ѧϰС������ͼװ�ý���ʵ�飨���ּг�װ����ȥ������Ӧһ��ʱ���ȡCƿ�е���Һ����ʵ�飬�����Һ��pH=12�����������ϣ�����NaClO��ҺpHΪ11�����ش��������⣺
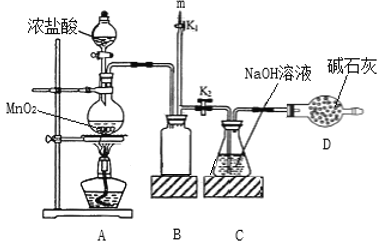
(1)װ��A�з�����Ӧ�����ӷ���ʽΪ_______��
(2)ʵ���������m���������ʢ________������NaOH ��Һ������ˮ���� ��ע������Ȼ���K1�ر�K2������ٲ��װ�á�
(3)�ⶨCƿ��Һ��NaClO������ʵ�鲽�����£�
����1����Cƿ��Һ���������ữ���������KI��Һ������ƿ�����ڰ�����ַ�Ӧ�����5~6�ε�����Һ��
����2�������θ�ȡ����1����Һ20mL����ƿ�У���0.1000mol��L��1 Na2S2O3 ����Һ�ζ����ζ��յ�ʱ���κ�Na2S2O3 ��Һ�����ƽ��ֵΪ16.00mL������֪��I2+2S2O32- =2I-+S4O62-��
�ٲ���1��Cƿ�з�����Ӧ�����ӷ���ʽΪ��______________________________��
�ڵζ��յ������Ϊ__________________________________________________��
��Cƿ��Һ��NaClO����Ϊ____________g��L��1��
����ʢNa2S2O3 ����Һ�ĵζ���δ��Na2S2O3 ����Һ��ϴ������Cƿ��Һ��NaClO����__________������ƫ��������ƫС����������������
���𰸡�MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O NaOH��Һ ClO-+2I-+2H+=Cl-+I2+H2O ���������һ��Na2S2O3��Һʱ����Һ����ɫ��Ϊ��ɫ�����ڰ�����ڲ���ɫ 2.98 ƫ��
Mn2++Cl2��+2H2O NaOH��Һ ClO-+2I-+2H+=Cl-+I2+H2O ���������һ��Na2S2O3��Һʱ����Һ����ɫ��Ϊ��ɫ�����ڰ�����ڲ���ɫ 2.98 ƫ��
��������
��Aװ���У�����MnO2��Ũ����ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O�����ɵ�Cl2����Bװ�ã���װ���Ƿ�����װ�ã�Ȼ��Cl2����Cװ���У���NaOH��Һ������ӦCl2+2NaOH=NaCl+NaClO+H2O�������Cl2�ü�ʯ�����գ��ɷ�ֹ��Ⱦ������ʵ�������K1�ر�K2������������ʢNaOH��Һ��ע�����У�����ٲ��װ�á�
MnCl2+Cl2��+2H2O�����ɵ�Cl2����Bװ�ã���װ���Ƿ�����װ�ã�Ȼ��Cl2����Cװ���У���NaOH��Һ������ӦCl2+2NaOH=NaCl+NaClO+H2O�������Cl2�ü�ʯ�����գ��ɷ�ֹ��Ⱦ������ʵ�������K1�ر�K2������������ʢNaOH��Һ��ע�����У�����ٲ��װ�á�
(1)װ��A�з���MnO2��Ũ����ķ�Ӧ����Ӧ�����ӷ���ʽΪMnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O����Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O������MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
(2)Ϊ�˷�ֹ�����Cl2�ݳ���Ⱦ������ʵ���������m���������ʢNaOH��Һ��ע������Ȼ���K1�ر�K2������ٲ��װ�á���Ϊ��NaOH��Һ��
(3)�ٲ���1��Cƿ��NaClO��������Һ����KI��Һ������Ӧ������NaCl��I2�ȣ����ӷ���ʽΪClO-+2I-+2H+=Cl-+I2+H2O����Ϊ��ClO-+2I-+2H+=Cl-+I2+H2O��
�ڷ�Ӧ���Cƿ�е���5~6�ε�����Һ����Һ����ɫ������0.1000mol��L��1 Na2S2O3 ����Һ�ζ����ζ��յ������Ϊ�����������һ��Na2S2O3��Һʱ����Һ����ɫ��Ϊ��ɫ�����ڰ�����ڲ���ɫ����Ϊ�����������һ��Na2S2O3��Һʱ����Һ����ɫ��Ϊ��ɫ�����ڰ�����ڲ���ɫ��
��Cƿ��Һ�з�����Ӧ�Ĺ�ϵʽΪ��NaClO����I2����2Na2S2O3��NaClO����Ϊ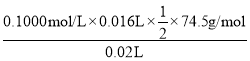 =2.98g��L��1����Ϊ��2.98��
=2.98g��L��1������2.98��
����ʢNa2S2O3 ����Һ�ĵζ���δ��Na2S2O3����Һ��ϴ����c(Na2S2O3)��С���������ƫ����˲��Cƿ��Һ��NaClO����ƫ��Ϊ��ƫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����800��ʱ����2L�ܱ������н�һ������NO��O2��Ϸ�����Ӧ��n(NO)��ʱ��ı仯���±���
ʱ��/S | 0 | 10 | 20 | 30 | 40 | 50 |
n(NO)/mol | 0.2 | 0.1 | 0.08 | 0.07 | 0.07 | 0.07 |
��ش��������⣺
(1)��O2��ʾ��0��20s�ڸ÷�Ӧ������Ϊ___________��
(2)�����µ�850����ƽ���n(NO)= n(NO2)����÷�Ӧ��______�ȷ�Ӧ�������������������
(3)���ı�ijһ����������ƽ��ʱn(NO)= 0.06 mol������˵����ȷ����_______��
A��ƽ��һ�������ƶ�
B���������������м�����һ������NO����
C���������������������
(4)������һ��������0.2molNO������������Ӧ���ﵽƽ��ʱ��÷ų�����akJ����ʱNOת����Ϊ80%��д���ڴ������¸÷�Ӧ���Ȼ�ѧ����ʽ_________��
(5)����ʱ��a��b������ʼ�����ȣ�������2molNO��1molO2��ƽ��ʱNO��ת����a___b��������������С��������������