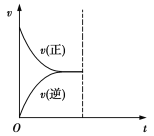
��Ŀ����
����Ŀ��������ԭ��һ����Ҫ�ķ�Ӧ��
��1��ijͬѧд������������ѧ����ʽ(δ��ƽ)
��N2O4��H2O��HNO3��HNO2 ��NO��HNO3��N2O3��H2O ��NH3��NO��HNO2��H2O
��������Ϊһ��������ʵ�ֵ��ǣ�����ţ�____________��
��2�����·�Ӧ��H2O2�����ֻ�ԭ�Ե��ǣ�����ţ�____________��H2O2�����������������ֻ�ԭ�Ե��ǣ�����ţ�____________���ӷ�Ӧ���ж�H2O2��Ag2O��K2CrO4��������ǿ������˳����_______________��
A��H2O2+2Fe2++2H+=2Fe3++2H2O
B��2H2O2=2H2O+O2��
C��Ag2O+H2O2=2Ag+O2��+H2O
D��3H2O2+Cr2(SO4)3+10KOH=2K2CrO4+3K2SO4+8H2O
E��H2O2+MnSO4=MnO2+H2SO4
��3������˫���ŷ����������·�Ӧ�е���ת�Ƶķ������Ŀ________������Ӧ����3.01��1023������ת�ƣ��������Ļ�ԭ�������ʵ���Ϊ___________��
2KMnO4��16HCl��Ũ��=2KCl��2MnCl2��5Cl2����8H2O
���𰸡� �� C B Ag2O��H2O2��K2CrO4 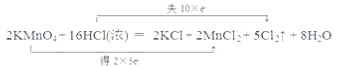 0.5mol
0.5mol
����������1������N2O4��NԪ�صĻ��ϼ������ߺͽ��ͣ������绯��Ӧ������ʵ�֣����е�NO�е�NԪ�صĻ��ϼ����ߣ�HNO3��NԪ�صĻ��ϼ۽��ͣ����ڹ��з�Ӧ������ʵ�֣�����NH3��NO��NԪ�صĻ��ϼ۶������ߣ�û�л��ϼ۽��͵�Ԫ�أ�������ʵ�֣��ʴ�Ϊ����
��2��A��H2O2��OԪ�صĻ��ϼ�ȫ��������ֻ����������ֻ���������ԣ������Դ�С��H2O2>Fe3+��B��H2O2��OԪ�صĻ��ϼۼ������ֽ��ͣ��ʼ������������ǻ�ԭ�������ֳ������Ժͻ�ԭ�ԣ�C��H2O2��OԪ�صĻ��ϼ�ȫ�����ߣ�ֻ����ԭ����ֻ���ֳ���ԭ�ԣ�Ag2O�������������������Դ�СΪAg2O��H2O2��D��H2O2��OԪ�صĻ��ϼ�ȫ�����ͣ�����������K2CrO4Ϊ����������������Դ�СΪH2O2��K2CrO4��E��H2O2��OԪ�صĻ��ϼ�û�б仯����û��������Ҳû�л�ԭ�ԡ����Ͽ�֪��H2O2�����ֻ�ԭ�Ե��Ƿ�ӦC��H2O2�����������������ֻ�ԭ�Ե��Ƿ�ӦB��H2O2��Ag2O��K2CrO4��������ǿ������˳����Ag2O��H2O2��K2CrO4
��3���÷�Ӧ��һ��������ԭ��Ӧ����˫���ű�ʾ���£�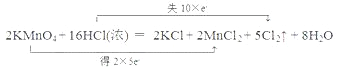 ��3.01��1023�����ӵ����ʵ���Ϊ0.5mol����ԭ��ΪHCl������ΪCl2��1molHCl����ԭ��ʧȥ1mol���ӣ�������ԭ����HCl��0.5mol��
��3.01��1023�����ӵ����ʵ���Ϊ0.5mol����ԭ��ΪHCl������ΪCl2��1molHCl����ԭ��ʧȥ1mol���ӣ�������ԭ����HCl��0.5mol��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���������⡿�����µ����[Ca(IO3)2]��һ�ְ�ɫ���壬����ˮ���������Ҵ������������ᣬ��Ŀǰ�㷺ʹ�õļ��ܲ������ܲ��Ƶ�����ʳƷ�ʹ������Ӽ������Ʊ�ԭ�����£�
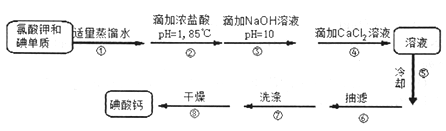
��.����Ƶ��Ʊ�
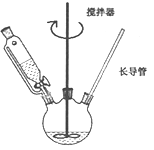
(1)ijͬѧ��Ƶ�ʵ��װ������ͼ��ʾ(�г�װ����ʡ��)������ڵļ��ȷ�ʽΪ_________��
(2)�������ȴ�ȵı�����Һû���������壬Ϊ�˵õ�������Բ�ȡ��һ�ִ�ʩ��________��
(3)�����ϴ�ӵľ��������____________��
��.��Ʒ���Ȳⶨ
ȷ��ȡ0.5000 g��Ʒ���ữ�ܽ⣬������250 mL,����ȡ��25.00 mL��������ƿ�У�����������KI��ַ�Ӧ����0.04000 mol��L-1�����������Һ�ζ����յ㣬�ظ����ϲ��裬�����ʵ���������£�
1 | 2 | 3 | |
�ζ���ʼ����/mL | 1.52 | 1.16 | 0.84 |
�ζ���ֹ����/mL | 31.50 | 31.18 | 30.84 |
��֪��2Na2S2O3 + I2= Na2S4O6 + 2NaI
(4)����KIʱ������Ӧ�����ӷ���ʽ��_____________��
(5)�ζ�ʱ���õ�ָʾ����_________________.
(6)�������Ʒ�Ĵ���Ϊ________________��