��Ŀ����
��12�֣���һ���¶��½�2 mol A��2 mol B������������ij2L�ܱ������У��������·�Ӧ��3A (g)��3B(g) 2C(g) ��2D(g)��2 min ĩ��Ӧ�ﵽƽ��״̬������0.8 mol D������д����հף�
2C(g) ��2D(g)��2 min ĩ��Ӧ�ﵽƽ��״̬������0.8 mol D������д����հף�
��1��B��ƽ��Ũ��Ϊ ��
��2��A��ת����Ϊ ��
��3����D��ʾ��ƽ����Ӧ����Ϊ ��
��4�������С��Ӧ�����ݻ����¶Ȳ��䣩��ʹѹǿ������ƽ����ϵ��C�����ʵ���Ũ�� ��C���������� ���������ƽ����Է������� �������������С�����䡱����
 2C(g) ��2D(g)��2 min ĩ��Ӧ�ﵽƽ��״̬������0.8 mol D������д����հף�
2C(g) ��2D(g)��2 min ĩ��Ӧ�ﵽƽ��״̬������0.8 mol D������д����հף���1��B��ƽ��Ũ��Ϊ ��
��2��A��ת����Ϊ ��
��3����D��ʾ��ƽ����Ӧ����Ϊ ��
��4�������С��Ӧ�����ݻ����¶Ȳ��䣩��ʹѹǿ������ƽ����ϵ��C�����ʵ���Ũ�� ��C���������� ���������ƽ����Է������� �������������С�����䡱����

��

��ϰ��ϵ�д�
�����Ŀ
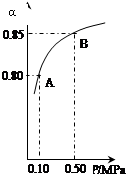
 NO2��CO ������˵����ȷ���ǣ� ��
NO2��CO ������˵����ȷ���ǣ� ��
 C(g)��D(g)���������������������仯ʱ���ٻ��������ܶȢ������������ѹǿ�ۻ�������ƽ����Է���������B�����ʵ���Ũ�� �ܱ����÷�Ӧһ���Ѵﵽƽ��״̬���ǣ� ��
C(g)��D(g)���������������������仯ʱ���ٻ��������ܶȢ������������ѹǿ�ۻ�������ƽ����Է���������B�����ʵ���Ũ�� �ܱ����÷�Ӧһ���Ѵﵽƽ��״̬���ǣ� ��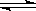 3KCl + Fe(SCN)3���ﵽƽ�����Һ�Ժ�ɫ����ʹƽ�������ƶ���������
3KCl + Fe(SCN)3���ﵽƽ�����Һ�Ժ�ɫ����ʹƽ�������ƶ��������� 2NO��O2���˷�Ӧ�ﵽƽ��״̬ʱ�ı�־��(����)
2NO��O2���˷�Ӧ�ﵽƽ��״̬ʱ�ı�־��(����)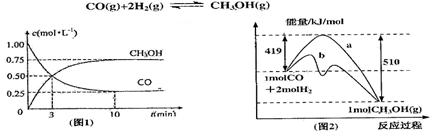
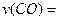 ��
��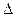 H>0������Ӧ��д����Ӧ���Ȼ�ѧ����ʽ ��ѡ�����˵Ĵ����� ����ܡ����ܡ����ı�÷�Ӧ�ķ�Ӧ�ȣ�
H>0������Ӧ��д����Ӧ���Ȼ�ѧ����ʽ ��ѡ�����˵Ĵ����� ����ܡ����ܡ����ı�÷�Ӧ�ķ�Ӧ�ȣ� ��ʽΪ ���¶����ߣ�ƽ�ⳣ��K ������������䡱��С������
��ʽΪ ���¶����ߣ�ƽ�ⳣ��K ������������䡱��С������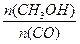 ����� ��
����� �� 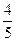 ,��N2��ת����Ϊ
,��N2��ת����Ϊ 2SO3(g)
2SO3(g)