��Ŀ����
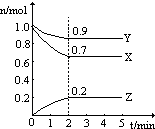
7��T��ʱ����һ��2L���ܱմ����У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף���1��д����X��Y��Z��ʾ�ĸ÷�Ӧ�Ļ�ѧ����ʽ3X+Y?2Z��
��2���ӷ�Ӧ��ʼ���մﵽƽ�⣬Z��ƽ����Ӧ����Ϊ0.05mol/��L��min����
��3���ı������������ܼӿ컯ѧ��Ӧ���ʵ���AD��
A�������¶� B����СX���� C����Сѹǿ D������Z������
���� ��1���������ʵ����ı仯�жϷ�Ӧ���������������ʵ����ı仯��֮�ȵ��ڻ�ѧ������֮����д����ʽ��
��2������v=$\frac{��c}{��t}$���㷴Ӧ����
��3���÷�Ӧ��һ����Ӧǰ�����������С�Ŀ��淴Ӧ������ѹǿ�������¶ȡ�����Ũ�ȶ�������Ӧ���ʣ�
��� �⣺��1����ͼ����Կ�������Ӧ��X��Y�����ʵ�����С��Z�����ʵ������࣬��X��YΪ��Ӧ�ZΪ�������n��X��=��1.0-0.7��mol=0.3mol����n��Y��=��1.0-0.9��mol=0.1mol����n��Z��=��0.2-0��mol=0.2mol����n��X������n��Y������n��Z��=0.3mol��0.1mol��0.2mol=3��1��2����Ӧ�Ļ�ѧ����ʽΪ��3X+Y?2Z��
�ʴ�Ϊ��3X+Y?2Z��
��2��Z��ƽ����Ӧ����v=$\frac{��c}{��t}$=$\frac{\frac{0.2mol}{2L}}{2min}$=0.05mol/��L��min�����ʴ�Ϊ��0.05mol/��L��min����
��3��A�������¶ȣ�����Ӱٷ����������Է�Ӧ���ʼӿ죬����ȷ��
B����СX����������Ũ�ȼ�С����λ����ڻ��������С�����Է�Ӧ���ʼ�С���ʴ���
C����Сѹǿ���������������Ũ�ȼ�С����λ����ڻ��������С�����Է�Ӧ���ʼ�С���ʴ���
D������Z������Ũ������λ����ڻ����������Ӧ���ʼӿ죬����ȷ��
��ѡAD��
���� ���⿼�鷽��ʽ��ȷ������Ӧ���ʼ��㼰��Ӧ����Ӱ�����أ����ؿ����������������֪�����ʵ����仯����������Ĺ�ϵ����Ӧ����Ӱ�����ؼ��ɽ����Ŀ�ѶȲ���

 ��������������������ϵ�д�
��������������������ϵ�д�| A�� | ��ϵͳ������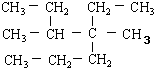 ������Ϊ4��5-����-4-�һ����� ������Ϊ4��5-����-4-�һ����� | |
| B�� | 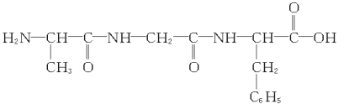 ��ȫˮ����Եõ�3�ְ����� ��ȫˮ����Եõ�3�ְ����� | |
| C�� | ʯ���ѽ����֬���������ɸ߷�����������С�������ʵĹ��� | |
| D�� | ������ ��һ�ȴ�����2�� ��һ�ȴ�����2�� |
| A�� | Ϊ��ֹ����е��ؽ�������Ⱦ������ˮ�壬Ӧ���������ϵ�ص��ۺ����ü��� | |
| B�� | �ߴ��ȵĶ�������㷺�����������ά����ǿ��ᡰ��·�� | |
| C�� | ��NO��N02Ϊ���ĵ����������γɹ⻯ѧ�����������һ����Ҫԭ�� | |
| D�� | FeCl3��Һ����Cu��Ӧ��������ʴ��ӡˢ��· |
| A�� | 11.2 L Cl2�к��е���ԭ���� | |
| B�� | ���³�ѹ�£�1.6 g CH4�к��е������� | |
| C�� | ��״���£�22.4 L H2O�к��е���ԭ���� | |
| D�� | 1 mol Na2SO4����ˮ��������Һ�к��е��������� |
| A�� | ��ȩ������ϩ���Ҷ���������Ϊ�ϳɾۺ���ĵ��� | |
| B�� | ��ѿ�������ǵ�ˮ�������������ǣ��ʶ��߾�Ϊ��ԭ�Զ��� | |
| C�� | �����ֶ��Ļ�Ϊͬ���칹�壬����ߵ�ˮ�����һ����ͬ | |
| D�� | ��Ȼֲ���ͳ�����һ���Һ̬��������ˮ���к㶨���۵㡢�е� |
 �ʻ���[Fe��CO��5]��ʹ�ϳɼ״��ͺϳɰ������������еĴ����ж���
�ʻ���[Fe��CO��5]��ʹ�ϳɼ״��ͺϳɰ������������еĴ����ж���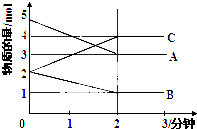 ij���淴Ӧ��0-2���ӽ��й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ�����������Ϊ2L����ͼ�����ݷ�������Ӧ��ʼ��2min��C��ƽ����Ӧ����Ϊ0.5mol/��L•min������ѧ����ʽΪ2A+B?2C��
ij���淴Ӧ��0-2���ӽ��й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ�����������Ϊ2L����ͼ�����ݷ�������Ӧ��ʼ��2min��C��ƽ����Ӧ����Ϊ0.5mol/��L•min������ѧ����ʽΪ2A+B?2C��