��Ŀ����
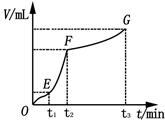
��8�֣���200mL 6mol��L-1�����м���һ�����Ĵ���CaCO3����������������ʱ��ı仯������ͼ��ʾ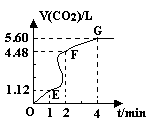 ������������ڱ�״���²ⶨ������ش��������⣺
������������ڱ�״���²ⶨ������ش��������⣺
��1����OE�εķ�Ӧ����Ϊv1��EF�εķ�Ӧ����Ϊv2��
FG�εķ�Ӧ����Ϊv3���� Ӱ��v1��v2��v3��Ӧ���ʵ������� ��
��2��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е��� ������ĸ��
| A������ˮ | B���Ȼ��ع��� | C���Ȼ�����Һ | D��Ũ���� |
 ��3������CaCO3������Ϊ ��
��3������CaCO3������Ϊ ����4������Ӧ��������Һ����ı仯���Բ��ƣ���EF���������ʾ�Ļ�ѧ��Ӧ����V��HCl��=______��
��1����Ӧ���¶ȣ���Ӧ���Ũ�ȣ�2��A C ��3��25g ��4��1��5mol��L-1��min-1
���������������1����OE��Ӱ�컯ѧ��Ӧ���ʵ������Ƿ�Ӧ���¶ȣ����ڸտ�ʼ��Ӧ���¶Ƚϵͣ��������ʽ��������ŷ�Ӧ�Ľ��У���Ӧ����ʹ��Һ���¶Ȳ������ߣ�������EF�εķ�Ӧ���ʴ��ӿ죬��Ӧ���Ͻ��У����ڷ�Ӧ�ﲻ�����ģ����������Ũ�Ȳ��Ͻ��͡���FG��Ӱ�컯ѧ��Ӧ���ʵ���Ҫ���ؾ��Ƿ�Ӧ���Ũ�ȡ���ʱŨ�Ƚϵ͡�������������С����Ӱ��v1��v2��v3��Ӧ���ʵ������Ƿ�Ӧ���¶Ⱥͷ�Ӧ���Ũ�ȡ���2��A�� ������ˮ��ʹ�����Ũ�Ƚ��ͣ���Ӧ���ʼ�������ȷ��B�����Ȼ��ع��壬����û�иı䷴Ӧ���Ũ�ȣ����ԶԻ�ѧ��Ӧ������Ӱ�죬����C�����Ȼ�����Һ����Һ�е�ˮ��������ϡ�����ã������Ũ�Ƚ��ͣ���Ӧ���ʼ�������ȷ��D����Ũ���ᣬ���ڷ�Ӧ���Ũ���������Ի�ѧ��Ӧ���ʴ��ӿ졣����3��n(HCl)=0��2L��6mol/L=1��2mol��n(CO2)=5��6L��22��4L/mol=0��25mol�����ݷ�Ӧ����ʽ��2HCl+ CaCO3=CaCl2+H2O+ CO2����֪��HCl��ȫ��Ӧ�ų���CO2������ڱ�״���µ����0��6mol��22��4L/mol=13��44L>5��6L��˵��HCl�������ų�����������Ӧ�ð���CaCO3�����㡣n(CaCO3) = n(CO2)= 0��25mol��m(CaCO3)= 25g��(4)��EF��n(CO2)=(4��48L��1��12L)�� 22��4L/mol= 0��15mol, ��n(HCl)= 2n(CO2) =0��3mol�� V��HCl��=(0��3mol��0��2L)��1min=1��5mol/( L��min)��
���㣺����Ӱ�컯ѧ��Ӧ���ʵ����ء���ѧ��Ӧ���ʵļ��㼰��Ӧ����һ���ǹ���ʱ�ļ����֪ʶ��

Ǧ���仯���﹤ҵ�������ճ�������зdz��㷺����;��
��1����Ŧ�Ʒ�����Ǧ������ط�Ӧ���Ȼ�ѧ����ʽ����:
2PbS(s)+3O2(g)=2PbO(s)+2SO2(g) ��H=" a" kJ/mol
PbS(s)+2PbO(s)=3Pb(s)+SO2(g) ��H=" b" kJ��mol-1
PbS(s)+PbSO4(s)=2Pb(s)+2SO2(g) ��H=" c" kJ��mol-1
��Ӧ3PbS(s) + 6O2(g) = 3PbSO4(s) ��H="kJ" ��mol-1(�ú�a,b ,c�Ĵ���ʽ��ʾ)��
��2����ԭ����Ǧ��������ӦPbO(s)+CO(g)  Pb(s) + CO2(g) ��H���÷�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���±�
Pb(s) + CO2(g) ��H���÷�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���±�
| �¶� | 300 | 727 | 1227 |
| lgK | 6.17 | 2.87 | 1.24 |
�ٸû�ԭ��Ӧ�ġ�H0(ѡ��:��>����<����=��)��
�ڵ�IgK=1����ʼʱֻͨ��CO(PbO����)����ƽ��ʱ�����������CO���������Ϊ ��
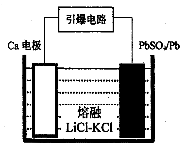
��3�������������������Ĺ�����Դͨ��Ca/PbSO4�ȵ�أ���װ����ͼ��ʾ���õ�������ĵ缫��ӦʽΪ ��
��4��PbI2:�������˹����ꡣȡһ������PbI2���壬������ˮ���Ƴ�t�汥����Һ��ȷ��ȡ25.00mLPbI2������Һ�ִμ��������ӽ�����֬RH+(����:2RH++PbI2=R2Pb+2H++2I-)����250ml�ྻ����ƿ��������Һ�����������ˮ��ϴ��֬������Һ�����ԣ���ϴ��Һһ��ʢ�ŵ���ƿ��(��ͼ)�������ָ̪ʾ������0.0025mol��L-1NaOH��Һ�ζ������ﵽ�ζ��յ�ʱ����ȥ����������Һ20.00mL���ɼ����t��ʱPbI2 KspΪ ��

��5��Ǧ����ɻ�����Ⱦ��ˮ��Һ�е�Ǧ������̬��Ҫ��6�֣�������pH��ϵ��ͼ��ʾ����Ǧ��ˮ�û���̿���д�����Ǧ��ȥ������pH��ϵ��ͼ��ʾ��


�ٳ����£�pH=6��7ʱ��Ǧ��̬��ת�������ӷ���ʽΪ ��
���û���̿������Ǧ��ȥ���ʽϸ�ʱ��Ǧ��ҪӦ�ô��� (��Ǧ��һ����̬�Ļ�ѧʽ)��̬��
��14�֣��о�CO2��CH4�ķ�Ӧʹ֮ת��ΪCO��H2���Լ���ȼ��Σ������������ЧӦ������Ҫ�����塣
��1����֪����2CO(g)+O2��g����2CO2(g)����H����566 kJ��mol-1
��2H2(g)+O2��g����2H2O(g)����H����484kJ��mol-1
��CH4(g)+2O2(g)��CO2(g)+2H2O(g)����H����802kJ��mol-1
��CH4(g)+CO2(g)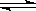 2CO(g)+2H2(g)����H������kJ��mol-1
2CO(g)+2H2(g)����H������kJ��mol-1
���������ܱ�������ͨ�����ʵ���Ũ�Ⱦ�Ϊ0.1mol����-1��CH4��CO2����һ�������·�����ӦCH4(g)+CO2(g)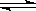 2CO(g)+2H2(g)�����CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
2CO(g)+2H2(g)�����CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��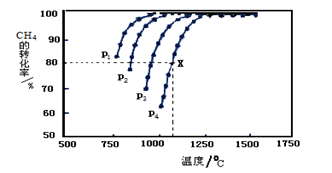
?��ͼ��֪��P1��P2�� P3��P4�ɴ�С��˳��������
?��ѹǿΪP4��1100��������£��÷�Ӧ5minʱ�ﵽƽ���X������CO��ʾ�÷�Ӧ������Ϊ �����¶��£���Ӧ��ƽ�ⳣ��Ϊ ����
��3��CO��H2�ڹ�ҵ�ϻ�����ͨ����ӦC(s)+H2O(g) 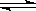 CO(g)+H2 (g)����ȡ��
CO(g)+H2 (g)����ȡ��
���ں��º����£�����ӷ�Ӧ���������ƽ�⣬���϶�ƽ���Ѵﵽ����
| A����ϵѹǿ���ٱ仯 | B��H2��CO�����ʵ���֮��Ϊ1 ��1 |
| C�����������ܶȱ��ֲ��� | D������ƽ����Է�������Ϊ15���ұ��ֲ��� |
2.2molH2(g)��һ������C(s)�������ʱ����ϵ��ѹ��ƽ���� ����������桱����Ӧ�����ƶ�����5minʱ�ﵽ�µ�ƽ�⣬������ͼ�л���2��5min������������ƽ����Է��������ı仯���ߡ�
��16�֣������еIJ��ֵ�Դ��O3�Ժ�ˮ��I������������O3����ͨ��NaI��Һ�н���ģ���о���
��1��O3��I��������I2�Ĺ�����3����Ӧ��ɣ�
��I��(aq)+ O3(g)==IO��(aq)+O2(g) ��H1
��IO��(aq)+H+(aq)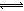 HOI(aq) ��H2
HOI(aq) ��H2
��HOI(aq)+ I��(aq)+ H+(aq)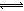 I2(aq)+H2O(l) ��H3
I2(aq)+H2O(l) ��H3
�ܷ�Ӧ�Ļ�ѧ����ʽΪ______���䷴Ӧ��H=______��
��2������Һ�д��ڻ�ѧƽ�⣺I2(aq)+I��(aq)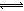 I3��(aq)����ƽ�ⳣ������ʽΪ_______��
I3��(aq)����ƽ�ⳣ������ʽΪ_______��
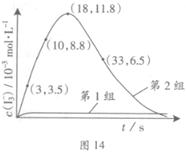
��3��Ϊ̽��Fe2+������I����Ӧ��Ӱ�죨��Ӧ��ϵ��ͼ13����ij�о�С��ⶨ����ʵ����I3��Ũ�Ⱥ���ϵpH�������ͼ14���±���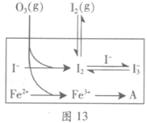
| ��� | ��Ӧ�� | ��ӦǰpH | ��Ӧ��pH |
| ��1�� | O3+ I�� | 5.2 | 11.0 |
| ��2�� | O3+ I��+ Fe2+ | 5.2 | 4.1 |
��ͼ13�е�AΪ ����Fe3+����A�Ĺ������������I����ת���ʣ�ԭ����_______��
�۵�2��ʵ�����18s��I3���½��������½���ֱ��ԭ���У�˫ѡ��______��
A��c(H+)��С B��c(I��)��С C��I2(g)�������� D��c(Fe3+)����
��4����ͼ14������3��18s�ڵ�2��ʵ��������I3����ƽ����Ӧ���ʣ�д��������̣����������λ��Ч���֣���
��7�֣�Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飺
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1 mol��L��1 FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��� | �Թ�A�����������ݲ����� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ��� |
��1����������ֽ�Ļ�ѧ����ʽΪ �������������� ��
��2��ʵ��ٵ�Ŀ����___________________________________________________��ʵ���еμ�FeCl3��Һ��Ŀ����________________________________________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_________________________����ʵ�������ṩ���Լ�����������
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ�������ж�OE��EF��FG�����У�___________�λ�ѧ��Ӧ������졣
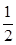 O2��g��
O2��g�� SO3��g����H����98 kJ��mol��1��
SO3��g����H����98 kJ��mol��1��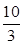 �����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol
�����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol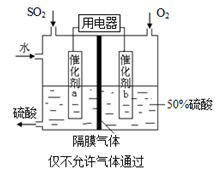
 x Q(g)+3R(g)����2min��ƽ�⣬����2.4molR�������Q��Ũ��Ϊ0.4mol/L����
x Q(g)+3R(g)����2min��ƽ�⣬����2.4molR�������Q��Ũ��Ϊ0.4mol/L���� 2C�ﵽƽ�⡣���ⶨ��ƽ��ʱc(A2)=0.5mol/L��c(B2)=0.1mol/L��c(C)=1.6mol/L����A2��B2��C����ʼŨ�ȷֱ�Ϊamol/L��bmol/L��cmol/L��
2C�ﵽƽ�⡣���ⶨ��ƽ��ʱc(A2)=0.5mol/L��c(B2)=0.1mol/L��c(C)=1.6mol/L����A2��B2��C����ʼŨ�ȷֱ�Ϊamol/L��bmol/L��cmol/L��