��Ŀ����
���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裬��֪
SO2��g����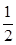 O2��g��
O2��g�� SO3��g����H����98 kJ��mol��1��
SO3��g����H����98 kJ��mol��1��
��1��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K��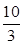 �����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol
�����ڴ��¶��£���100 L�ĺ����ܱ������У�����3.0 mol
SO2(g)��16.0 mol O2(g)��3.0 mol SO3(g)����Ӧ��ʼʱv�� v�������������������������
��2��һ���¶��£���һ�����������Ϊ2 L���ܱ������г���2.0 mol SO2��1.0 molO2���ﵽƽ��������Ϊ1.6 L����SO2��ƽ��ת����Ϊ ��
��3���ڣ�2���еķ�Ӧ�ﵽƽ��ı�������������ʹSO2(g)ƽ��Ũ�ȱ�ԭ����С���� ������ĸ����
A�����¶Ⱥ�����������䣬����1.0 mol O2
B�����¶Ⱥ�������ѹǿ���䣬����1.0 mol SO3
C�����¶�
D�ƶ�����ѹ������
��4��������ͼ��ʾװ�ã��õ绯ѧԭ���������ᣬд��ͨ��O2�缫�ĵ缫��ӦʽΪ ��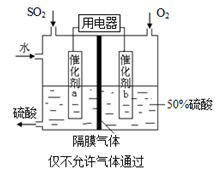
��5��Ϊ�ȶ�����������������Һ��Ũ��Ӧά�ֲ��䣬��ͨ��SO2��ˮ��������Ϊ ��
��1����
��2��60%
��3��A��C
��4��O2��4e����4H+��2H2O
��5��16:29
����

��8�֣���200mL 6mol��L-1�����м���һ�����Ĵ���CaCO3����������������ʱ��ı仯������ͼ��ʾ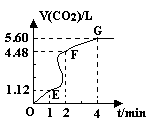 ������������ڱ�״���²ⶨ������ش��������⣺
������������ڱ�״���²ⶨ������ش��������⣺
��1����OE�εķ�Ӧ����Ϊv1��EF�εķ�Ӧ����Ϊv2��
FG�εķ�Ӧ����Ϊv3���� Ӱ��v1��v2��v3��Ӧ���ʵ������� ��
��2��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е��� ������ĸ��
| A������ˮ | B���Ȼ��ع��� | C���Ȼ�����Һ | D��Ũ���� |
 ��3������CaCO3������Ϊ ��
��3������CaCO3������Ϊ ����4������Ӧ��������Һ����ı仯���Բ��ƣ���EF���������ʾ�Ļ�ѧ��Ӧ����V��HCl��=______��
�״�ȼ�Ϸ�Ϊ�״����ͺͼ״����͡���ҵ�Ϻϳɼ״��ķ����ܶࡣ
��1��һ�������·�����Ӧ��
��CO2��g��+3H2��g����CH3OH��g��+H2O��g�� ��H1
��2CO��g��+O2��g�� ��2CO2��g�� ��H2
��2H2��g��+O2��g����2H2O��g�� ��H3
��CO��g�� + 2H2��g��  CH3OH��g������H�� ��
CH3OH��g������H�� ��
��2�����ݻ�Ϊ2L���ܱ������н��з�Ӧ�� CO��g��+2H2��g�� CH3OH��g�� �������������䣬��300���500��ʱ�����ʵ���n��CH3OH���뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0����>��<��=����
CH3OH��g�� �������������䣬��300���500��ʱ�����ʵ���n��CH3OH���뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0����>��<��=����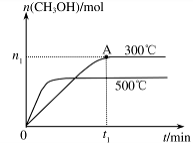
��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________������ĸ����
| A����������� |
| B�������¶� |
| C�������¶� |
| D��ʹ�ú��ʵĴ��� |
��4��CH4��H2O�ڴ������淢����ӦCH4+H2O
 CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣���
CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣�����5���Լ״�Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺

��B���ĵ缫��ӦʽΪ ��
�����ø�ȼ�ϵ������Դ����ʯī���缫�������ͭ��Һ������·��ת��1mole-ʱ��ʵ�������ĵļ״��������������ϴ���ԭ���� ��
��6��25��ʱ������Ƶ�Ksp=4.0��10-8,̼��Ƶ�Ksp=2.5��10-9����20ml̼��Ƶı�����Һ����μ���8.0��10-4 mol��L-1�IJ������Һ20ml���ܷ�������� ����ܡ�����
ij̽��С���ý������ֱ���ϡ�����ϡ���ᷴӦ�ķ����о������벻ͬ�ᷴӦ�IJ��켰Ӱ�췴Ӧ�ٶȵ����ء�
ʵ��ҩƷ��2.0 mol/L���ᡢ4.0 mol/L���ᡢ2.0 mol/L���ᡢ4.0 mol/L���ᡢ��ͬ��С����Ƭ������(������������Ĥ���ѳ�ȥ)��ÿ��ʵ��������������Ϊ50.0 mL������������Ϊ9.0 g��
��.��ͬѧ����Ƭ�ֱ��ϡ���ᡢϡ���ᷴӦ��ʵ�鼰���������£�
| ��Ӧ���� (����) | 1 | 2 | 5 | 15 | 20 |
| 4.0 mol/L ���� | �������� | �϶����� | �������� | ��Ӧ���� | ��Ƭ�ľ� |
| 2.0 mol/L ���� | ���������� | ������ ���� | �������� | ||
| 4.0 mol/L ���� | ���������� | �������� | �бȽ����������� | ||
��ش��������⣺
(1)д���������ᷴӦ�����ӷ���ʽ�� ��
(2)��Ӧ��1��15min�ڣ���������ķ�Ӧ������������ԭ���� ��
(3)��������̽��������ϡ�����ϡ����ķ�Ӧ���ʴ��ڲ����ԭ�����ܶ�ԭ��������Щ�������룺 (д��һ�ּ���)��
��.��ͬѧ���������Ӱ�췴Ӧ�������ص�ʵ�顣�������ʵ��Ŀ�İ�����ͬѧ�������ʵ����Ʊ���
| ʵ��Ŀ�� | ʵ�� ��� | �¶� /�� | �������� ��̬ | ����Ũ�� /mol��L��1 |
| 1.ʵ��ٺ͢�̽������Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻 2��ʵ��ٺ͢�̽���¶ȶԸ÷�Ӧ���ʵ�Ӱ�죻 3��ʵ��ٺ͢�̽���������(��Ƭ�� ����)�Ը÷�Ӧ���ʵ�Ӱ�� | �� | 25 | ��Ƭ | 4.0 |
| �� | | | | |
| �� | | | | |
| �� | | | |
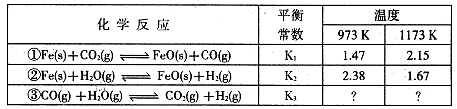
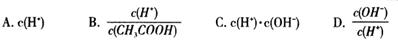
 CO(g)��H2(g)��
CO(g)��H2(g)�� H2(g)��CO2(g)��
H2(g)��CO2(g)�� 2NH3(g)����H��0�����ѹǿ��ʱ��ͼ����ͼ�ף����p2��0.6p1����ʱ�¶�����ʼ�¶���ͬ���ڴﵽƽ��ǰijһʱ��(t1)�����ı�һ���������õ�����ͼ��
2NH3(g)����H��0�����ѹǿ��ʱ��ͼ����ͼ�ף����p2��0.6p1����ʱ�¶�����ʼ�¶���ͬ���ڴﵽƽ��ǰijһʱ��(t1)�����ı�һ���������õ�����ͼ��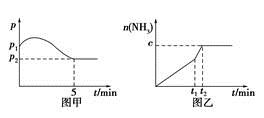
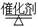 CO(g)��3H2(g)�Ʊ�CO��H2����һ��������1 L���ܱ������г���0.3 mol H2O��0.2 mol CH4�����H2(g)��CH4(g)�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ��0��4 s�ڣ���CO(g)��ʾ�ķ�Ӧ����Ϊ____________��
CO(g)��3H2(g)�Ʊ�CO��H2����һ��������1 L���ܱ������г���0.3 mol H2O��0.2 mol CH4�����H2(g)��CH4(g)�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ��0��4 s�ڣ���CO(g)��ʾ�ķ�Ӧ����Ϊ____________�� xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��c(A):c(B)=3:5��C��ƽ����Ӧ������0.1 mol/(L��min)����
xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��c(A):c(B)=3:5��C��ƽ����Ӧ������0.1 mol/(L��min)����