��Ŀ����
����Ŀ��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飺
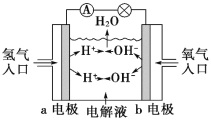
(1)��һ�������л���A�����������г��ȼ��,ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L(��״����)��������ʵ�ʵ��ʽ��____________��
(2)�������Dzⶨ���л����������Է����������õ���ͼ1��ʾ������ͼ��������Է�������Ϊ_____________�������ʵķ���ʽ��_____________��
(3)��Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ��_____________��
(4)�˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ(�ź�)�����ݷ�ֵ(�ź�)����ȷ����������ԭ�ӵ��������Ŀ��������ȼ���(ClCH2OCH3����2����ԭ��)�ĺ˴Ź���������ͼ2��ʾ��
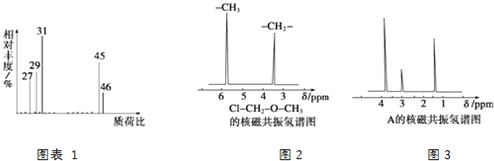
���ⶨ���л���A�ĺ˴Ź�������ͼ��ͼ3��ʾ����A�Ľṹ��ʽΪ_____________��
���𰸡� C2H6O 46 C2H6O CH3CH2OH��CH3OCH3 CH3CH2OH
��������(1)����ˮ��������̼���������ʵ���������ԭ���غ���������C��H��Oԭ����Ŀ֮��ȷ�����ʽ��
(2)�����ʺɱȿ�֪��Է�������Ϊ46��������ʽȷ������ʽ��
(3)���ݷ���ʽ��д���ܵĽṹ��ʽ��
(4)�л���A�ĺ˴Ź�����������3�����շ壬˵����3�����ʲ�ͬ��Hԭ�ӣ���Ϸ���ʽȷ���ṹ��ʽ��
(1)5.4gH2O�����ʵ���=![]() =0.3mol����n(H)=0.6mol��8.8gCO2�����ʵ���=
=0.3mol����n(H)=0.6mol��8.8gCO2�����ʵ���=![]() =0.2mol����n(C)=0.2mol��6.72LO2�����ʵ���
=0.2mol����n(C)=0.2mol��6.72LO2�����ʵ���![]() =0.3mol����OԪ���غ��֪�л����к���n(O)=0.3mol+0.2mol��2-0.3mol��2=0.1mol���������n(C)��n(H)��n(O)=2��6��1�������ʽΪ��C2H6O���ʴ�Ϊ��C2H6O��
=0.3mol����OԪ���غ��֪�л����к���n(O)=0.3mol+0.2mol��2-0.3mol��2=0.1mol���������n(C)��n(H)��n(O)=2��6��1�������ʽΪ��C2H6O���ʴ�Ϊ��C2H6O��
(2)�����ʺɱȿ�֪��Է�������Ϊ46�����ʽ��Է�������Ҳ��46���ʷ���ʽΪ��C2H6O���ʴ�Ϊ��46��C2H6O��
(3)����ʽΪC2H6O�Ŀ��ܽṹ��ʽΪ��CH3CH2OH��CH3-O-CH3���ʴ�Ϊ��CH3CH2OH��CH3-O-CH3��
(4)�л���A�ĺ˴Ź�����������3�����շ壬˵����3�����ʲ�ͬ��Hԭ�ӣ���A�ĽṹΪ��CH3CH2OH���ʴ�Ϊ��CH3CH2OH��

 ��У����ϵ�д�
��У����ϵ�д�